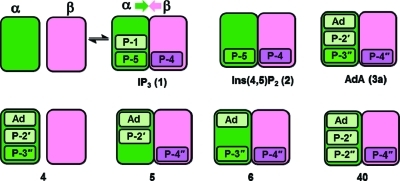
Figure 7.
Schematic representation of IP3R activation by inositol phosphates and adenophostin analogues. IP3 (1) and Ins(4,5)P2 (2) activate IP3R by engaging residues in the α- (green) and β- (pink) domains of the IBC, stabilizing a closed conformation that favors opening of the C-terminal Ca2+ channel.45 Phosphate groups may have strong (dark green) or weaker (light green) interactions with the α-domain. Molecular modeling suggests that Ada (3a) has additional interactions (light green) with the α-domain of the IBC, accounting for the greater potency of AdA. 4″-Dephospho-AdA (4) is essentially inactive because it cannot form effective β-domain interactions. However, 3″-dephospho-Ada (5) and 2′-dephospho-AdA (6) retain activity because they can effectively engage both domains, even though 5 does not contain a vicinal bisphosphate pair. The previously unexplained and relatively potent activity of AdA regiosomer 40 can now be explained by analogy with 5. P = phosphate group, and Ad = adenine.