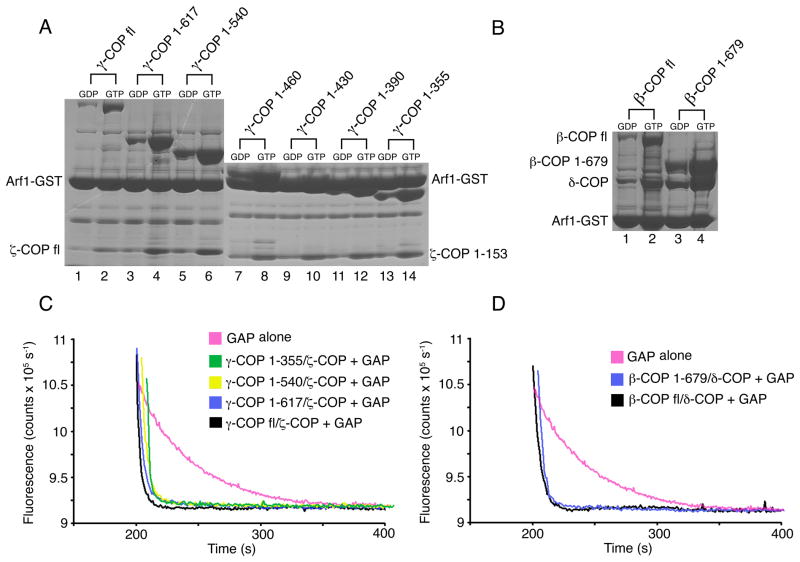
Figure 2. Identification of Arf1-binding sites on βδ/γζ-COP.
(A) GTP-dependent binding of Arf1 to γζ-COP dimers. The pull-down assay was utilized to test the Arf1-binding capacity of truncated forms of γ-COP. In the experiments, γ-COP was present in a dimer with full-length ζ-COP (lanes 1–6) or truncated (1–153) ζ-COP (lanes 7–14). All of the dimeric proteins, from the longest to the shortest forms (left to right), bind to Arf1.
(B) GTP-dependent binding of Arf1 to βδ-COP dimers. Arf1 binds to full-length βδ-COP (lane 2) and to a βδ-COP dimer lacking the β-COP appendage domain (lane 4).
(C) Various forms of γζ-COP, including the minimal γζ-COP dimer (γ-COP 1–355 and ζ-COP 1–153) identified in (A), are fully active in the fluorescence assay—that is, all synergize with GAP to catalyze GTP hydrolysis on Arf1. For clarity, the curves have been offset incrementally along the x-axis.
(D) The βδ-COP dimer can also synergize with GAP to catalyze GTP hydrolysis on Arf1. Curves have been offset incrementally along the x-axis.