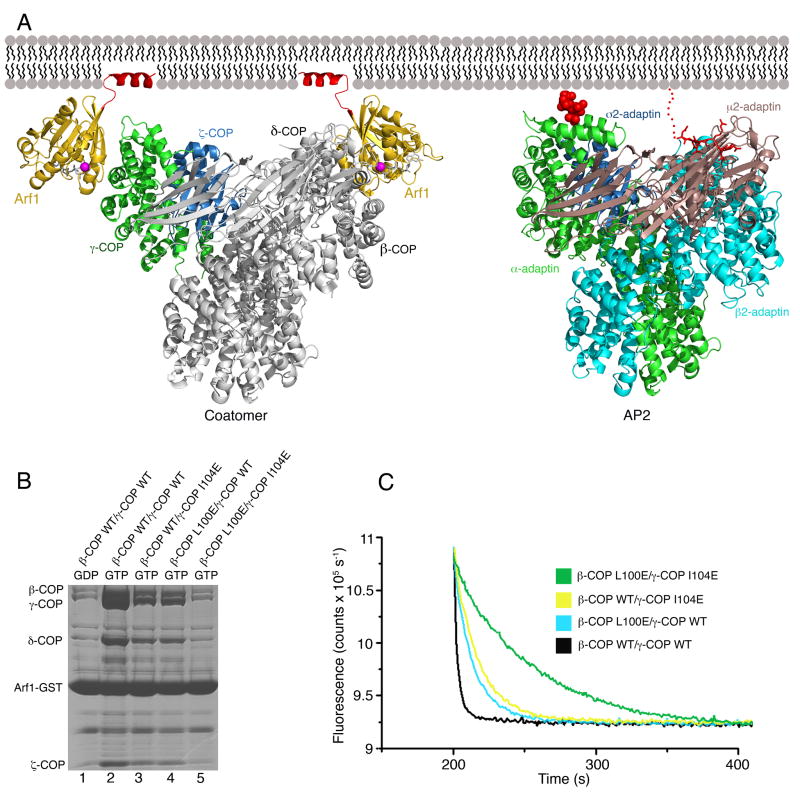
Figure 6. Model for Membrane Recruitment of Coatomer.
(A) Left panel shows a composite model of βδ/γζ-COP bound to membrane via two molecules of Arf1-GTP. The γζ-COP/Arf1 crystal structure is colored (following the scheme used in Figure 3) as is the second molecule of Arf1. The remainder of βδ/γζ-COP, in grey, is modeled based on homology with the AP2 adaptor complex; specifically, we used the crystal structure of the open conformation of AP2 (Jackson et al., 2010). The N-terminal amphipathic α helices of Arf1 (colored red) are modeled in their expected locations as membrane anchors. The right panel shows the structure of the open form of AP2 (as described by Jackson et al. [2010]) modeled in an interaction with membrane via a PtdIns4,5P2 headgroup (van der Waals spheres colored red) bound to the primary site on α-AP and a YxxΦ cargo sorting motif (stick representation colored red) that interacts with the μ2-AP subunit. For clarity, we have drawn AP2 in the same orientation as βδ/γζ-COP; this required only a slight shift of the membrane-bound orientation proposed by Jackson et al. (2010). See also Figure S2.
(B) Effects of mutations in the Arf1-binding sites of full-length βδ/γζ-COP complex, measured using the pull-down assay. Single mutations reduce but do not abolish Arf1 interaction (lanes 3 and 4), whereas a double mutation binds to Arf1 at background levels (compare lane 5 to lane 1).
(C) The effects of single and double mutations in the βδ/γζ-COP complex on GTP hydrolysis in the fluorescence assay.