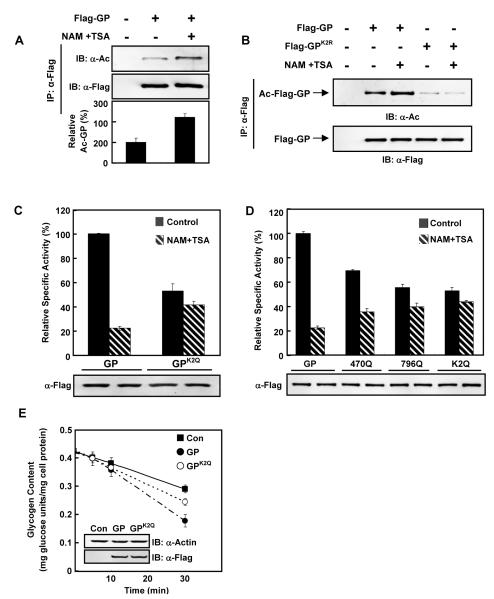
Figure 1. GP activity is negatively regulated by acetylation.
(A) GP acetylation is increased by deacetylase inhibitor. Flag-tagged GP was expressed in Chang’s cells with or without nicotinamide and trichostatin A (NAM+TSA) treatment. Acetylation levels of Flag-beads purified GP were blotted with pan-anti-acetyllysine antibody (α-AcK). IB and IP denote immunoblotting and immunoprecipitation, respectively.
(B) K470 and K796 are the major acetylation sites in GP. Ectopically expressed Flag-GP and GPK2R in Chang’s cells were immunopurified and immuno-blotted with pan-anti-acetyllysine antibody.
(C) GP catalytic activity is negatively regulated by acetylation. Both wild type GP and GPK2Q were expressed in Chang’s liver cells with or without NAM+TSA treatment. Catalytic activity of affinity purified GP proteins were determined and normalized to protein levels. Activity of wild type GP under no treatment condition was set as 100%.
(D) The acetylation target K470 and K796 are important for GP inhibition by the deacetylase inhibitor treatment. GP, GPK470Q, GPK796Q and GPK2Q were each expressed in Chang’s liver cells with (hatched bars) or without (solid bars) NAM+TSA treatment as indicated. Specific activity of each purified enzyme was determined.
(E) Wild-type GP degrades glycogen faster than K2Q mutant. Wild type GP and mutant GPK2Q were expressed at similar levels in Chang’s liver cells maintained in regular DMEM medium. The medium was replaced by glucose-free DMEM at time 0 and the glycogen degradation rate was measured. All error bars represent standard deviation (SD). n = 3 for each experimental group.