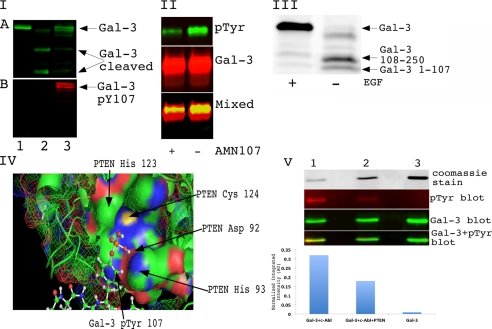
FIGURE 1.
Phosphorylation of galectin-3 on Tyr-107 regulates its cleavage by PSA. Panel I, phosphorylation on Tyr-107 (pY107) blocks the cleavage of Gal-3 by PSA in vitro. An equal amount of recombinant galectin-3 wild type was loaded on the gel. Lane 1, galectin-3. Lane 2, PSA-treated galectin-3. Lane 3, galectin-3 was phosphorylated by c-Abl and treated with PSA. The reaction was stopped by adding sample buffer, resolved on a 10% SDS-PAGE gel, and immunoblotted using anti-galectin-3 antibody (A) or anti-Tyr(P) (pTyr) antibody (B). Panel II, tyrosine phosphorylation of galectin-3 in vivo is blocked by c-Abl inhibitor. PC3M cells were treated with 150 ng/ml EGF and 50 ng/ml PDGF for 8 h. One plate was also treated with 1 μm AMN107 (c-Abl inhibitor). Conditioned media were collected, and galectin-3 was immunoprecipitated with TIB166 antibody. Samples were resolved using 10% SDS-PAGE gel and immunoblotted with anti-phosphotyrosine antibody (top panel) anti-galectin-3 (HL31) (middle panel). The bottom panel represents overlapping Tyr(P) and galectin-3 blots loaded and run on an SDS-PAGE gel. Panel III, EGF treatment blocks cleavage of galectin-3 in conditioned medium of co-cultured LNCaP and PC3M cells. LNCaP and PC3M cells were co-cultured for 24 h. One plate was treated with 150 ng/ml EGF for 8 h. Conditioned medium was collected and concentrated using Millipore filters with a 3-kDa cutoff. 50 μg of total protein was loaded and run on a gradient (4–20%) SDS-PAGE gel. After transfer, membrane was blotted with polyclonal HL31 antibody, which can recognize multiple epitopes on galectin-3, for 2 h at room temperature. Panel IV, docking of Gal-3 phosphorylated on Tyr-107 with PTEN. Autodock4 (Scripps Research Institute) was used to dock the phosphorylated version of Gal-3 and PTEN. His-123, Cys-124, Asp-92, and His-93 represent amino acids from the catalytic active site and form the PTEN HCXXGXXR motif, the Cys-124 and Arg-130 residues are essential for catalysis, and the His-123 residue is important for the conformation of the P loop. Panel V, dephosphorylation of galectin-3 Tyr(P)-107 with PTEN. Lane 1, wild type recombinant galectin-3 phosphorylated with c-Abl. Lane 2, phosphorylated galectin-3 treated with PTEN for 2 h at 30 °C. Lane 3, recombinant untreated galectin-3. the top panel represents 15% of the sample mixture run on 10% SDS-PAGE and visualized with Coomassie Blue stain. The rest of the samples were resolved using 10% SDS-PAGE gel and immunoblotted with anti-phosphotyrosine antibody (second panel) and anti-galectin-3 (HL31) antibody (third panel). The bottom panel represents overlapping Tyr(P) and galectin-3 blots. The normalized integrated intensity was calculated as band integrated intensity. AU, arbitrary units.