Background: There is no known receptor for the nucleotide AMP.
Results: AMP directly activates the adenosine A1 receptor (A1R) but not the adenosine A2B receptor.
Conclusion: A1R is a receptor for adenosine and AMP.
Significance: The diverse physiological effects of AMP could be due to direct, ectonucleotidase-independent activation of A1R.
Keywords: Adenosine, Adenosine Receptor, Calcium Imaging, G Protein-coupled Receptors (GPCR), Nucleoside Nucleotide Analogs
Abstract
Numerous receptors for ATP, ADP, and adenosine exist; however, it is currently unknown whether a receptor for the related nucleotide adenosine 5′-monophosphate (AMP) exists. Using a novel cell-based assay to visualize adenosine receptor activation in real time, we found that AMP and a non-hydrolyzable AMP analog (deoxyadenosine 5′-monophosphonate, ACP) directly activated the adenosine A1 receptor (A1R). In contrast, AMP only activated the adenosine A2B receptor (A2BR) after hydrolysis to adenosine by ecto-5′-nucleotidase (NT5E, CD73) or prostatic acid phosphatase (PAP, ACPP). Adenosine and AMP were equipotent human A1R agonists in our real-time assay and in a cAMP accumulation assay. ACP also depressed cAMP levels in mouse cortical neurons through activation of endogenous A1R. Non-selective purinergic receptor antagonists (pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid and suramin) did not block adenosine- or AMP-evoked activation. Moreover, mutation of His-251 in the human A1R ligand binding pocket reduced AMP potency without affecting adenosine potency. In contrast, mutation of a different binding pocket residue (His-278) eliminated responses to AMP and to adenosine. Taken together, our study indicates that the physiologically relevant nucleotide AMP is a full agonist of A1R. In addition, our study suggests that some of the physiological effects of AMP may be direct, and not indirect through ectonucleotidases that hydrolyze this nucleotide to adenosine.
Introduction
Adenosine and adenine nucleotides regulate diverse physiological processes (1, 2). Adenosine activates four distinct G protein-coupled receptors, the so-called P1 purinergic receptors: adenosine A1 receptor (A1R,2 ADORA1), adenosine A2A receptor (A2AR, ADORA2A), adenosine A2B receptor (A2BR, ADORA2B), and adenosine A3 receptor (A3R, ADORA3). A1R and A3R are Gi/o-coupled and inhibit adenylate cyclase when activated, whereas A2AR and A2BR are Gs-coupled and stimulate adenylate cyclase.
Although mammals have numerous P2 purinergic receptors for ATP and ADP, no receptor for their hydrolysis product (AMP) has been definitively identified. GPR80/GPR99 was originally classified as an adenosine and AMP receptor (3); however, this finding has now been discounted (4, 5). AMP has diverse physiological effects, suggesting that a receptor for AMP could exist (6–15).
Complicating studies with AMP is the fact that cells express multiple enzymes that hydrolyze extracellular AMP to adenosine, including ecto-5′-nucleotidase (NT5E, CD73), prostatic acid phosphatase (PAP, ACPP), and several alkaline phosphatases (16–19). Genetic deletion or pharmacological inhibition of individual ectonucleotidases reduces, but does not always eliminate, the physiological effects of AMP (6, 8, 11, 13, 20). In addition, the most commonly used ectonucleotidase inhibitor α,β-methylene-ADP (αβ-met-ADP) inhibits NT5E, but it does not inhibit PAP (11).
Many of the physiological effects of AMP are lost in A1R knock-out mice or can be blocked with adenosine receptor antagonists (6, 8, 11, 13–15), suggesting that adenosine is the active ligand. However, given the challenges associated with inhibiting all ectonucleotidases in complex tissues (20), direct activation of adenosine receptors by AMP cannot be easily ruled out.
Extracellular AMP originates from multiple endogenous sources (16), and nucleotide release and hydrolysis can be rapid (20, 21). Endogenous AMP could thus directly modulate diverse adenosine receptor-dependent processes, including cardiovascular disease, cancer, neurotransmission, and gliotransmission (1, 2, 22–24).
To rigorously study direct and indirect effects of AMP on adenosine receptors, we developed a novel cell-based assay utilizing chimeric G proteins to visualize human (h)A1R and hA2BR activation in real time and at single cell resolution. We also used a non-hydrolyzable AMP analog to rule out the effects of AMP hydrolysis. Surprisingly, we found that AMP directly activated hA1R, but not hA2BR, and activation was independent of hydrolysis to adenosine. Thus, our study indicates that A1R is a receptor for the naturally occurring nucleotide AMP as well as a receptor for adenosine.
EXPERIMENTAL PROCEDURES
Animal Care and Use
All procedures involving vertebrate animals were approved by the Institutional Animal Care and Use Committee.
Calcium Imaging
HEK293 cells were grown on polylysine-coated glass bottom culture dishes (MatTek Corp., P35G-0-10-C) in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were transfected with Lipofectamine Plus (Invitrogen) in DMEM containing 1% fetal bovine serum, which was replaced with fresh growth medium after 4 h. The total amount of DNA per transfection was adjusted to 1 μg by adding pcDNA 3.1(+). 100 ng of pCS-Venus was included in each transfection to identify transfected cells. Following transfection (∼24 h), cells were washed two times in Hanks' balanced salt solution assay buffer (Invitrogen catalogue number 14025, supplemented with 9 mm HEPES, 11 mm d-glucose, 0.1% fatty acid-free bovine serum albumin, pH 7.3), and then loaded for 1 h at room temperature with 2 μm Fura-2 AM (Invitrogen, F-1221) and 0.02% Pluronic F-127 (Invitrogen, P3000-MP) in assay buffer. Cells were washed three times with assay buffer, incubated for 30 min at room temperature, and imaged on a Nikon Eclipse Ti microscope.
A Sutter DG-4 light source (excitation 340 nm/380 nm; emission 510 nm) and Andor Clara CCD camera were used to image calcium responses. Assay buffer was refreshed immediately prior to imaging. Antagonists were added in assay buffer 3 min prior to imaging. Starting solution was aspirated, and agonist solution was added after 40 s of baseline imaging. We manually pipetted and aspirated solutions for all calcium imaging experiments. Only cells that expressed visible Venus protein, did not saturate the camera at a 40-ms exposure time, and had low (<0.6) baseline Fura-2 ratios were analyzed. 500-ms excitation at 340 nm and 250-ms excitation at 380 nm were used for all experiments.
Calcium responses were analyzed in two ways. To create real-time response profiles, the Fura-2 fluorescence intensity ratio (340 nm/380 nm) at each time point was averaged over all transfected cells in each condition and then normalized relative to the average baseline fluorescence ratio before agonist addition. Calcium responses were also quantified by calculating the area under the curve (AUC) extending 1 min from agonist addition, relative to the baseline fluorescence ratio, on a cell-by-cell basis. AUC values were then averaged over all cells in each condition. Calcium response profiles and AUC data are presented as mean ± S.E. To create dose-response curves, GraphPad Prism was used to fit a variable slope dose-response equation to the average AUC values for each agonist concentration. Detailed descriptions of materials and protocols can be found in the supplemental Experimental Procedures.
RESULTS
Chimeric G Proteins Can Be Used to Visualize Adenosine Receptor Activation in Real Time
Activation of Gi-coupled A1R or Gs-coupled A2BR is typically quantified by measuring ligand-evoked decreases or increases in intracellular cAMP, respectively. However, these assays entail lysing cells at different times after stimulation; hence these assays have limited temporal resolution and no cellular resolution. In an effort to develop a real-time readout of adenosine receptor activation, we co-transfected HEK293 cells with vectors encoding hA1R and Gαq-i5 (Gqi), a chimeric G protein that couples Gi-coupled receptors to phospholipase C (PLC) activation and calcium mobilization (supplemental Fig. S1) (25). In parallel, we co-transfected HEK293 cells with vectors encoding hA2BR and chimeric Gαq-s5 (Gqs) to couple hA2BR to PLC and calcium mobilization (supplemental Fig. S1) (26). As controls, we found that adenosine (1 mm) did not evoke calcium mobilization in untransfected HEK293 cells or in cells expressing hA1R alone, hA2BR alone, Gqi alone, or Gqs alone. However, a saturating concentration of adenosine (1 mm) evoked rapid onset calcium responses that slowly decayed in cells co-expressing hA1R + Gqi or hA2BR + Gqs (Fig. 1, A and B), indicating that adenosine receptor activation can be monitored in real time at cellular resolution.
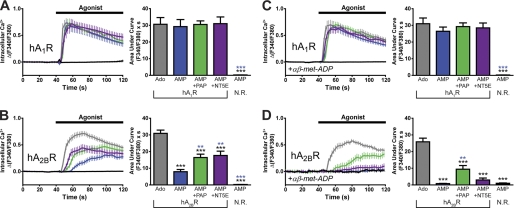
FIGURE 1.
AMP directly activates hA1R, whereas AMP activates hA2BR indirectly via ectonucleotidase-catalyzed hydrolysis to adenosine. A–D, calcium mobilization responses in HEK293 cells expressing (A and C) Gqi ± hA1R or (B and D) Gqs ± hA2BR. Ado, adenosine; N. R., no receptor. C and D, cells were incubated with the competitive NT5E inhibitor αβ-met-ADP (10 μm) for 3 min and then stimulated with 1 mm agonist in the presence of 10 μm αβ-met-ADP. Black, 1 mm AMP in the absence of a transfected adenosine receptor, but in the presence of the respective chimeric G protein. AUC measurements extended for 1 min from agonist addition. Paired t tests were used to compare AUC data. Black asterisks, statistically significant difference when compared with adenosine stimulation. Blue asterisks, statistically significant difference when compared with AMP stimulation (in receptor-expressing cells). *, p < 0.05, **, p < 0.005, ***, p < 0.0005. All data are the average of two experiments performed in duplicate. n = 20–74 cells per condition. All data, including calcium traces, are presented as means ± S.E.
AMP Activates hA1R Independent of Ectonucleotidases, whereas hA2BR Activation Is Ectonucleotidase-dependent
Because no AMP receptors have been definitively identified, we next hypothesized that AMP would only mobilize calcium in adenosine receptor-expressing cells after hydrolysis to adenosine by PAP or NT5E ectonucleotidases (supplemental Fig. S1). To test this hypothesis, we co-transfected cells with hA1R + Gqi vectors along with the membrane-bound ectonucleotidases PAP or NT5E. These ectonucleotidases were enzymatically active when expressed in HEK293 cells (see supplemental Experimental Procedures). To our initial surprise, 1 mm AMP evoked calcium responses in hA1R + Gqi-expressing cells that were indistinguishable in onset and magnitude from calcium responses evoked by 1 mm adenosine, and co-expression of PAP or NT5E did not modify AMP-evoked responses (Fig. 1A). Similar results were observed in cells co-expressing mouse A1R + Gqi (supplemental Fig. S2). These AMP-evoked calcium responses were dependent on overexpressed A1R as AMP had no effect in HEK293 cells when Gqi was expressed alone (Fig. 1A, supplemental Fig. S2). Rapid activation of A1R was not due to contaminating levels of adenosine (adenosine was undetectable in our AMP stock solution based on high performance liquid chromatography analysis). Instead, our data suggested that a nucleotide (AMP) could directly activate the adenosine A1 receptor.
In contrast, adenosine (Fig. 1B, gray) and AMP (Fig. 1B, blue) evoked kinetically distinct calcium responses in hA2BR + Gqs co-expressing HEK293 cells. Specifically, adenosine evoked a rapid onset calcium response that peaked shortly after agonist addition, whereas AMP elicited a gradual calcium response that began ∼15 s after agonist addition. Co-expression of PAP or NT5E significantly augmented the speed and magnitude of the AMP response (Fig. 1B, green and purple), suggesting that these enzymes accelerated the hydrolysis of AMP to adenosine. Although HEK293 cells endogenously express hA2BR (3, 27), AMP did not evoke calcium responses in cells expressing Gqs alone (Fig. 1B, black). Taken together, these data suggest that AMP only activates hA2BR indirectly following hydrolysis to adenosine.
To directly test whether AMP activates hA1R or hA2BR as a result of ectonucleotidase activity, we repeated our experiments in the presence of αβ-met-ADP, a high potency (Ki = 5 nm) NT5E inhibitor (28). We found that αβ-met-ADP did not inhibit adenosine- or AMP-evoked calcium responses in cells co-expressing hA1R + Gqi (with or without overexpressed ectonucleotidases) (compare Fig. 1A with Fig. 1C), further suggesting that AMP stimulates hA1R directly. In contrast, 10 μm αβ-met-ADP completely inhibited the calcium response caused by AMP in hA2BR + Gqs-expressing cells (Fig. 1D, blue) but did not inhibit the adenosine-evoked calcium response (Fig. 1D, gray). αβ-met-ADP also significantly reduced the calcium response caused by AMP in NT5E co-expressing cells (Fig. 1D, purple) and marginally inhibited the calcium response caused by AMP in PAP co-expressing cells (Fig. 1D, green).
Non-hydrolyzable AMP Analog Activates hA1R but Not hA2BR
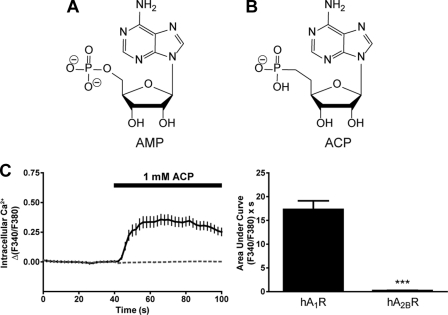
We next sought to determine whether ACP, a non-hydrolyzable analog of AMP (28), could also activate hA1R (Fig. 2, A and B). We found that 1 mm ACP evoked a rapid onset calcium response in hA1R + Gqi-expressing cells (Fig. 2C, solid line) but no response in hA2BR + Gqs-expressing cells (Fig. 2C, dashed line). ACP, AMP, and adenosine also evoked rapid calcium responses in COS7 cells expressing hA1R + Gqi, but not Gqi alone, indicating that all of these compounds activate hA1R when expressed in a different cell line (supplemental Fig. S3).
FIGURE 2.
Non-hydrolyzable AMP analog activates hA1R but not hA2BR. A and B, structures of AMP (A) and the non-hydrolyzable analog ACP (B) at physiological pH. C, calcium mobilization responses elicited by 1 mm ACP in HEK293 cells expressing (solid line) hA1R + Gqi or (dashed line) hA2BR + Gqs. Cells expressing Gqi alone did not respond to ACP. AUC measurements extended for 1 min from agonist addition. Paired t tests were used to compare AUC data, ***, p < 0.0005. Data are the average of one (hA2BR) or two (hA1R) experiments performed in duplicate. n = 28–73 cells per condition. All data, including calcium traces, are presented as means ± S.E.
Adenosine and AMP Are Equipotent hA1R Agonists
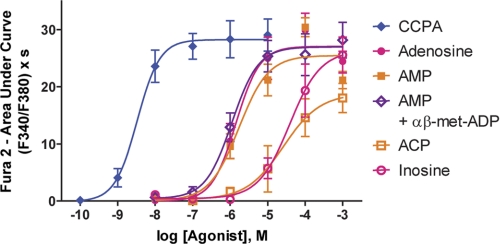
Because our experiments above were performed using a single concentration of agonist, we next performed dose-response experiments to determine agonist potency in hA1R + Gqi-expressing cells (Fig. 3). We found that adenosine stimulated calcium responses with an EC50 of 1.41 μm (Table 1), a value consistent with previously published measurements (12, 29). Interestingly, the EC50 and Emax of AMP in the absence or presence of αβ-met-ADP (to ensure that AMP was not hydrolyzed by endogenous NT5E) were not significantly different from those of adenosine (Fig. 3, Table 1). The non-hydrolyzable analog ACP had an EC50 of 26.1 μm, 15-fold higher than AMP. This reduced potency relative to AMP could reflect the charge difference between the phosphonate and phosphate groups (monoanionic versus dianionic at neutral pH, respectively, Fig. 2). The adenosine deamination product inosine had an EC50 of 38.1 μm, 27-fold higher than adenosine. The high potency A1R agonist 2-chloro-N6-cyclopentyladenosine (CCPA) evoked calcium responses with an EC50 of 3.32 nm. The Hill slopes of all dose responses were near 1.
FIGURE 3.
Dose-response curves for adenosine, AMP, and related analogs at hA1R. Calcium mobilization was measured in HEK293 cells expressing hA1R + Gqi and stimulated with the indicated compounds. For the AMP + αβ-met-ADP condition, cells were incubated with 10 μm αβ-met-ADP for 3 min and then stimulated with AMP in the presence of 10 μm αβ-met-ADP. AUC measurements extended for 1 min from agonist addition. All experiments were performed in duplicate. n = 19–45 cells per condition. All data are presented as means ± S.E.
TABLE 1.
hA1R dose responses for Ca2+ mobilization assay and GloSensor assay
| Agonist | Ca2+ mobilization |
Inhibition of cAMP accumulation (EC50) | |
|---|---|---|---|
| EC50 | Emaxa | ||
| μm | % | μm | |
| CCPA | 0.00332 | 104.3 ± 2.0 | 0.0171 |
| Adenosine | 1.41 | 100 | |
| AMP | 1.69 | 94.1 ± 14.1 | 0.816 |
| AMPb | 1.11 | 99.6 ± 3.3 | 0.601 |
| ACP | 26.1 | 69.4 ± 3.2 | 4.95 |
| Inosine | 38.1 | 97.2 ± 3.6 | 3.22 |
a Relative to adenosine.
b In the presence of 10 μm αβ-met-ADP.
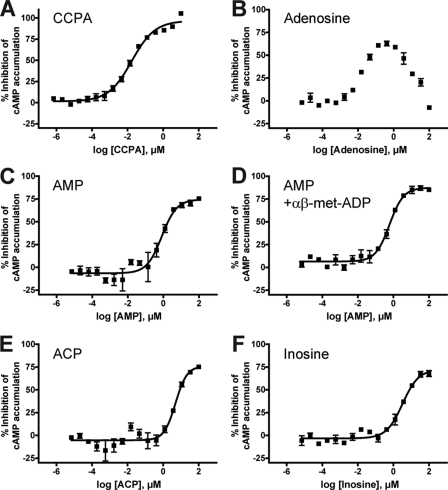
To test the potency of these compounds in an assay that does not depend on the use of chimeric G proteins, we utilized a modified Promega GloSensor assay (Fig. 4, see supplemental Experimental Procedures for assay details). This assay measures agonist effects on isoproterenol-evoked cAMP accumulation using a luminescent reporter construct and is conducted in cells containing only endogenous G proteins. When stimulating hA1R-expressing cells with adenosine, we observed a bimodal dose response, with adenosine inhibiting cAMP accumulation at low concentrations and stimulating cAMP accumulation at high concentrations (Fig. 4B). The latter response was likely due to activation of Gs-coupled A2 receptors, which HEK293 cells endogenously express (3, 27). Indeed, adenosine stimulated additional cAMP accumulation in cells transfected with empty vector (supplemental Fig. S4B). This bimodal response prevented us from obtaining an EC50 value for adenosine.
FIGURE 4.
Adenosine, AMP, and related analogs inhibit cAMP accumulation in hA1R-expressing cells. A–F, HEK293T cells were co-transfected with a vector encoding hA1R and GloSensor 22F plasmid and then stimulated with CCPA (A), adenosine (B), AMP (C) or AMP in the presence of 10 μm αβ-met-ADP (D), ACP (E), or inosine (F). Cells were incubated with test compound for 10 min, and then 175 nm (−)-isoproterenol was added for 7 min to stimulate cAMP accumulation. Following incubation, GloSensor cAMP reagent was added, and luminescence was measured. Data were normalized such that 100% inhibition is equal to the response at the maximal concentration of CCPA and 0% is equal to the response from isoproterenol alone. All experiments were performed in duplicate. All data are presented as means ± S.D.
In contrast, CCPA, AMP, ACP, and inosine exclusively inhibited cAMP accumulation in hA1R-expressing cells (Fig. 4, A and C–F) but had no effects in cells transfected with empty vector (supplemental Fig. S4, A and C–E). Incubation with 100 ng/ml pertussis toxin for 16 h after transfection abolished the A1R-mediated inhibition of cAMP accumulation by adenosine, AMP, and CCPA (data not shown), confirming that signaling was mediated through endogenous Gi proteins. The rank order of agonist potency was similar between cAMP accumulation assays and calcium mobilization assays (Table 1). The EC50 values of AMP were not significantly different in the presence or absence of 10 μm αβ-met-ADP (Fig. 4, C and D), further indicating that AMP stimulates hA1R/Gi-coupled signaling directly and independent of hydrolysis to adenosine. Moreover, AMP did not produce a bimodal response like adenosine, arguing that the signaling effects of AMP were specific to hA1R and not due to hydrolysis to adenosine.
ACP Inhibits Forskolin-evoked cAMP Accumulation in Embryonic Mouse Cortical Neurons
Because the experiments above relied upon overexpression of adenosine receptors, to further assess physiological relevance, we next sought to determine whether the non-hydrolyzable AMP analog (ACP) could activate native A1R and native downstream signaling components in a primary cell type. We selected mouse embryonic cortical neurons because AMP directly activates mouse A1R (supplemental Fig. S2), mouse A1R is highly expressed (Fig. 5A), and A1R activation regulates the physiology of cortical neurons (24). We utilized a cAMP ELISA assay to measure the second messenger that is downstream of Gi-coupled mouse A1R. In addition, we used ACP to ensure that no adenosine was produced from endogenously expressed ectonucleotidases.
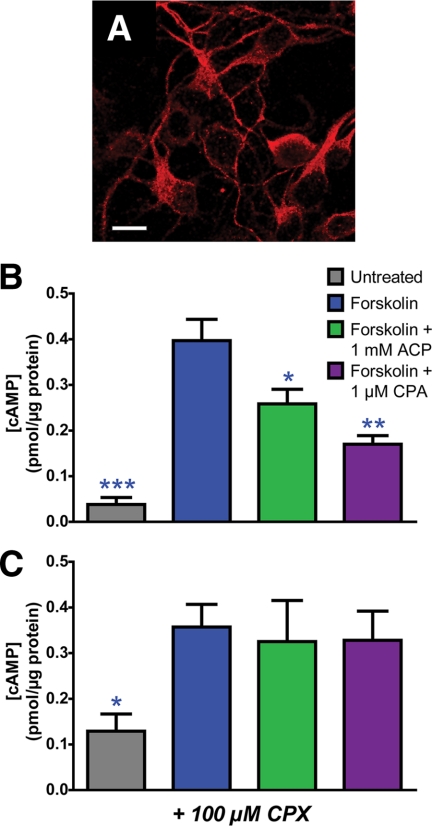
FIGURE 5.
ACP inhibits cAMP accumulation in mouse embryonic cortical neurons. Embryonic cortical neurons were dissociated and plated at about embryonic day 16.5. A, confocal image of cortical neurons immunostained with an anti-A1R antibody. Scale bar = 10 μm. B and C, after 1 day in vitro, neurons were incubated for 30 min with 1 mm ACP or 1 μm cyclopentyladenosine (CPA) in the absence (B) or presence (C) of 100 μm CPX. Neurons were then stimulated with 10 μm forskolin for 15 min, washed, and lysed. Cell lysates were then applied to a cAMP ELISA assay according to the manufacturer's instructions. cAMP concentrations were normalized to total protein using a BCA protein assay. Asterisks, statistically significant difference when compared with forskolin stimulation alone. *, p < 0.05, **, p < 0.005, ***, p < 0.0005. Data are the average of four (− CPX) or two (+ CPX) experiments performed in duplicate. All data are presented as means ± S.E.
Stimulation of cortical neurons with the adenylyl cyclase activator forskolin (10 μm) for 15 min increased intracellular cAMP concentration by 10-fold when compared with baseline (Fig. 5B, blue). The concentration of cAMP in neurons treated with 1 mm ACP for 30 min prior to forskolin stimulation was decreased 34.9% when compared with neurons treated with forskolin alone (Fig. 5B, green), and cAMP concentration decreased 57.2% in neurons treated with 1 μm N6-cyclopentyladenosine (Fig. 5B, purple), a high potency A1R agonist. In addition, the potent A1R antagonist CPX (100 μm) completely blocked the effects of ACP and cyclopentyladenosine, as evidenced by no decrease in cAMP concentration when compared with forskolin alone (Fig. 5C). Thus, these data indicate that a non-hydrolyzable AMP analog can directly activate endogenous signaling pathways downstream of endogenously expressed A1R (i.e. in cells that were not subjected to any genetic manipulation).
AMP Stimulates hA1R Independent of P2Y Receptors
HEK293/T cells express multiple P2Y receptors (5, 30), and P2Y receptors can heterodimerize with A1R, imparting a P2Y-like pharmacology on A1R (31–33). In addition, P2Y receptors can be stimulated by AMP analogs but not by AMP (34). Thus, we evaluated whether AMP-evoked calcium responses in hA1R-expressing cells could be blocked with non-selective P2Y antagonists (pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid or suramin). We found that stimulation of untransfected HEK293 cells with 10 μm ATP elicited a rapid calcium response (supplemental Fig. S5A, gray). This response was blocked completely by 100 μm pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (supplemental Fig. S5A, blue) and by 100 μm suramin (supplemental Fig. S5A, green). However, the same concentrations of pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid or suramin did not block calcium responses in hA1R + Gqi-expressing cells that were stimulated with 10 μm adenosine (supplemental Fig. S5B) or 10 μm AMP (supplemental Fig. S5C). These data thus indicate that AMP signals directly through hA1R, independent of P2Y receptor activity.
His-251 and His-278 in Ligand Binding Pocket Are Required for AMP to Directly Activate hA1R
The crystal structures of an adenosine receptor (hA2AR) co-crystallized with adenosine and an adenosine analog were recently reported (35, 36). From these structural views of the ligand binding pocket, we selected two positively charged histidine residues (His-251 and His-278; conserved in hA1R) as possibly important for interacting with the negatively charged phosphate of AMP. We then mutagenized each of these histidine residues to a non-polar residue (alanine) to generate hA1R-H251A and hA1R-H278A. We confirmed that each mutant receptor was expressed and membrane-localized to the same extent as wild-type hA1R (Fig. 6, A–C).
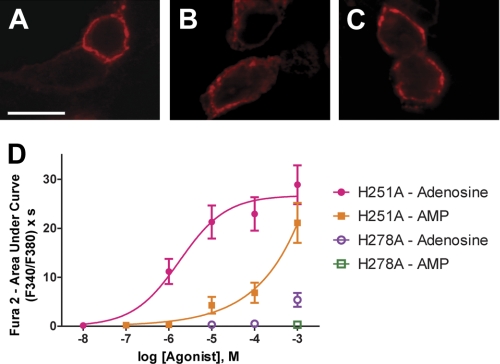
FIGURE 6.
Expression and activity of hA1R point mutants. A–C, confocal images of HEK293 cells expressing wild-type hA1R (A), hA1R-H251A (B), or hA1R-H278A (C) and immunostained with anti-A1R antibodies. Untransfected HEK293 cells in the same field of view were not immunostained, confirming antibody specificity. Scale bar = 10 μm. D, calcium mobilization in HEK293 cells co-expressing the indicated hA1R mutant and Gqi and then stimulated with increasing concentrations of the indicated compounds. For all conditions, cells were incubated with 10 μm αβ-met-ADP for 3 min and then stimulated with agonist in the presence of 10 μm αβ-met-ADP. AUC measurements extended for 1 min from agonist addition. All experiments were performed in duplicate, except H278A-AMP, which was performed in triplicate. n = 17–49 cells per condition. All data are presented as means ± S.E.
Next, we measured adenosine and AMP potency at the mutant receptors with our calcium mobilization assay (Fig. 6D). We performed these experiments in the presence of 10 μm αβ-met-ADP to prevent AMP from slowly being hydrolyzed to adenosine. In cells expressing hA1R-H251A + Gqi, adenosine stimulated calcium mobilization with an EC50 of 1.80 μm, essentially identical to what we observed when stimulating wild-type hA1R with adenosine (Table 1). Likewise, mutation of His-251 did not affect binding of a radiolabeled agonist to bovine A1R (37). In contrast, the potency of AMP in hA1R-H251A + Gqi-expressing cells was drastically reduced, so much so that we could not obtain a complete dose response. In cells expressing hA1R-H278A + Gqi, we observed a very weak response after stimulation with 1 mm adenosine, but no response at lower concentrations. Furthermore, stimulation with 1 mm AMP elicited no response. This is also consistent with a previous study, which showed that mutation of His-278 abolished agonist binding (37). Taken together, our results provide compelling evidence that adenosine and AMP activate hA1R directly, with activation requiring an agonist binding pocket residue (His-278) that is conserved in all adenosine receptors. Furthermore, the positively charged His-251 residue is critical for activation of hA1R by a negatively charged nucleotide (AMP) but not by adenosine. Importantly, these mutagenesis experiments conclusively rule out the possibility that adenosine and AMP stimulate calcium mobilization through any other receptor in HEK293 cells.
DISCUSSION
Prior to our study, it was unknown whether a receptor for AMP existed. An older study suggested that AMP might act directly and indirectly on adenosine receptors (14); however, the investigators did not fully inhibit the multiple ectonucleotidases that are now known to hydrolyze AMP to adenosine (16, 18, 19). In addition, AMP has never been evaluated as an agonist with cloned adenosine receptors, possibly because all previous assays of adenosine receptor activation required relatively long incubation periods with agonist, causing uncertainty as to whether AMP or its hydrolysis product (adenosine) was the active compound.
Using a novel cell-based assay that allowed for real-time visualization of adenosine receptor activation, we found that AMP directly activated hA1R, independent of hydrolysis. In support of this conclusion, we found that AMP activated hA1R in HEK293 cells as effectively as adenosine, even after inhibition of the main ectonucleotidase in HEK293 cells. Furthermore, a non-hydrolyzable analog of AMP also activated hA1R in heterologous cells and in primary neurons, showing that activation was not due to hydrolysis to adenosine. Our data thus provide the first direct evidence that hA1R is a receptor for the naturally occurring nucleotide AMP and argue for reclassification of A1R as an adenosine and nucleotide (AMP) receptor.
Inbe et al. (3) previously reported that GPR80/GPR99 was a receptor for adenosine and AMP, although others could not reproduce this result (4, 5). As suggested by Abbracchio et al. (4), GPR80/GPR99 may have been misidentified as a purinergic receptor because HEK293 cells (the cells used in the GPR80/GPR99 study and our present study) endogenously express P2Y receptors in addition to A2AR and A2BR. Alternatively, heteromeric interactions between GPR80/GPR99 and endogenous purinergic receptors could hypothetically impart GPR80/GPR99 with a novel pharmacological profile.
Neither of these hypothetical possibilities explains why AMP activated hA1R in our assays. The HEK293 cells we used do contain A2 receptors (as evidenced by stimulation of cAMP production in cells transfected only with GloSensor plasmid (supplemental Fig. S4)) and P2Y receptors (as evidenced by ATP-evoked, P2Y antagonist-sensitive calcium responses (supplemental Fig. S5)). However, our data with P2Y antagonists rule out the possibility that AMP signaled through P2Y receptors. In addition, point mutations in hA1R shifted or eliminated responses to AMP, providing strong evidence that AMP signaled directly through hA1R and not through any other receptor in HEK293 cells. AMP also directly stimulated hA1R when expressed in a different mammalian cell line (COS7 cells (supplemental Fig. S3)).
Our findings were also not an artifact of using a chimeric G protein to couple hA1R to calcium mobilization. Indeed, we found that AMP (±αβ-met-ADP) and ACP activated hA1R when coupled to endogenous Gi proteins using the GloSensor cAMP accumulation assay and that this effect could be blocked by Gi-specific disruption with pertussis toxin. Our findings were not an artifact of overexpressing A1R, as ACP inhibited forskolin-induced cAMP accumulation in mouse cortical neurons that contain only native A1R and downstream signaling components.
There is a large amount of structure-activity data with 5′-substituted adenosine analogs, all of which indicate that A1R is tolerant of bulky and negatively charged groups at the 5′-position (38–40). This includes 5′-ester, 5′-carbamoyl, 5′-halogen, and 5′-sulfide derivatives of adenosine analogs, many of which are low nanomolar agonists of A1R (41–44). Despite this extensive literature with unnatural analogs, it is surprising that the most biologically relevant substitution, a 5′-phosphate, has never, to our knowledge, been directly tested as an A1R agonist.
Our data also revealed that a different adenosine receptor, hA2BR, is not activated by AMP or the non-hydrolyzable analog ACP (Fig. S6). Instead, hA2BR was only activated indirectly, following hydrolysis to adenosine. These results shed light on seemingly conflicting reports of AMP acting directly as well as indirectly on adenosine receptors in some tissues but only indirectly via conversion to adenosine in other tissues (6, 12–14, 45, 46). Our data indicate that it is important to determine which adenosine receptor is activated when AMP is used as the ligand. If A2BR is activated, the signaling effects of AMP should be indirect and fully dependent on ectonucleotidases. In contrast, if A1R is activated, the signaling effects of AMP could be direct and indirect, with the level of direct activation dependent on AMP stability and ectonucleotidase levels.
We found that a non-hydrolyzable phosphonate analog of AMP could activate hA1R. Interestingly, other ectonucleotidase-resistant phosphonate analogs of AMP reportedly activate P2X receptors and have cardioprotective activity in vivo (47, 48). Although it was suggested that the cardioprotective effects of these AMP analogs were due to P2X activation, P2X involvement was never directly tested in vivo with antagonists or knock-out mice. Given that A1R agonists also have cardioprotective effects (2), it is equally possible that the cardioprotective effects of these phosphonate analogs, and possibly AMP itself, are A1R-mediated and not P2X-mediated.
Our findings also have implications for AMP-based prodrugs that were designed to be full agonists for A2AR only after hydrolysis by ectonucleotidases (49). Given our results and the extensive structure-activity data with substitutions at the 5′-position, these AMP prodrugs may display a complex pharmacology with direct A1R activation combined with indirect, hydrolysis-dependent A2AR activation. Clearly, it will be important to rigorously evaluate the extent to which these and other AMP-based prodrugs activate A1R independent of hydrolysis. This will require pharmacologically or genetically eliminating all of the AMP hydrolytic enzymes in a given tissue, a potentially daunting task given that numerous ectonucleotidases are present in complex tissues and are difficult to completely eliminate experimentally (20).
Supplementary Material
Acknowledgments
We thank Vincent Setola and Adam Cheely for technical assistance with the GloSensor assay, Eduardo R. Lazarowski for assessing the purity of our AMP stock using HPLC, and Jaeda Coutinho-Budd for assistance with cortical neuron dissections. Dr. Bruce Conklin (the University of California, San Francisco) and Sang-Kyou Han (the University of California, San Diego) generously provided chimeric G protein expression plasmids.
This work was supported, in whole or in part, by National Institutes of Health Grants R01NS060725 and R01NS067688 from the NINDS (to M. J. Z.).

This article contains supplemental Experimental Procedures and Figs. S1–S6.
- A1R
- adenosine A1 receptor
- A2AR
- adenosine A2A receptor
- A2BR
- adenosine A2B receptor
- A3R
- adenosine A3 receptor
- ACP
- deoxyadenosine 5′-monophosphonate
- NT5E
- ecto-5′-nucleotidase
- PAP
- prostatic acid phosphatase
- αβ-met-ADP
- α,β-methylene-ADP
- AUC
- area under the curve
- CCPA
- 2-chloro-N6-cyclopentyladenosine
- CPX
- 8-cyclopentyl-1,3-dipropylxanthine
- h
- human.
REFERENCES
- 1. Burnstock G. (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797 [DOI] [PubMed] [Google Scholar]
- 2. Jacobson K. A., Gao Z. G. (2006) Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 5, 247–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inbe H., Watanabe S., Miyawaki M., Tanabe E., Encinas J. A. (2004) Identification and characterization of a cell surface receptor, P2Y15, for AMP and adenosine. J. Biol. Chem. 279, 19790–19799 [DOI] [PubMed] [Google Scholar]
- 4. Abbracchio M. P., Burnstock G., Boeynaems J. M., Barnard E. A., Boyer J. L., Kennedy C., Miras-Portugal M. T., King B. F., Gachet C., Jacobson K. A., Weisman G. A. (2005) The recently deorphanized GPR80 (GPR99) proposed to be the P2Y15 receptor is not a genuine P2Y receptor. Trends Pharmacol. Sci. 26, 8–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qi A. D., Harden T. K., Nicholas R. A. (2004) GPR80/99, proposed to be the P2Y15 receptor activated by adenosine and AMP, is not a P2Y receptor. Purinergic Signal 1, 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruns R. F. (1980) Adenosine receptor activation by adenine nucleotides requires conversion of the nucleotides to adenosine. Naunyn Schmiedebergs Arch. Pharmacol. 315, 5–13 [DOI] [PubMed] [Google Scholar]
- 7. Salter M. W., Henry J. L. (1985) Effects of adenosine 5′-monophosphate and adenosine 5′-triphosphate on functionally identified units in the cat spinal dorsal horn: evidence for a differential effect of adenosine 5′-triphosphate on nociceptive versus non-nociceptive units. Neuroscience 15, 815–825 [DOI] [PubMed] [Google Scholar]
- 8. Dunwiddie T. V., Diao L., Proctor W. R. (1997) Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J. Neurosci. 17, 7673–7682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao N., Hu H. Z., Liu S., Gao C., Xia Y., Wood J. D. (2007) Stimulation of adenosine A1 and A2A receptors by AMP in the submucosal plexus of guinea pig small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G492–G500 [DOI] [PubMed] [Google Scholar]
- 10. Daniels I. S., Zhang J., O'Brien W. G., 3rd, Tao Z., Miki T., Zhao Z., Blackburn M. R., Lee C. C. (2010) A role of erythrocytes in adenosine monophosphate initiation of hypometabolism in mammals. J. Biol. Chem. 285, 20716–20723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sowa N. A., Voss M. K., Zylka M. J. (2010) Recombinant ecto-5′-nucleotidase (CD73) has long lasting antinociceptive effects that are dependent on adenosine A1 receptor activation. Mol. Pain 6, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ragazzi E., Wu S. N., Shryock J., Belardinelli L. (1991) Electrophysiological and receptor binding studies to assess activation of the cardiac adenosine receptor by adenine nucleotides. Circ. Res. 68, 1035–1044 [DOI] [PubMed] [Google Scholar]
- 13. Patterson S. L., Sluka K. A., Arnold M. A. (2001) A novel transverse push-pull microprobe: in vitro characterization and in vivo demonstration of the enzymatic production of adenosine in the spinal cord dorsal horn. J. Neurochem. 76, 234–246 [DOI] [PubMed] [Google Scholar]
- 14. Moody C. J., Meghji P., Burnstock G. (1984) Stimulation of P1-purinoceptors by ATP depends partly on its conversion to AMP and adenosine and partly on direct action. Eur. J. Pharmacol. 97, 47–54 [DOI] [PubMed] [Google Scholar]
- 15. Mustafa S. J., Nadeem A., Fan M., Zhong H., Belardinelli L., Zeng D. (2007) Effect of a specific and selective A2B adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J. Pharmacol. Exp. Ther. 320, 1246–1251 [DOI] [PubMed] [Google Scholar]
- 16. Zylka M. J. (2011) Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol. Med. 17, 188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zylka M. J., Sowa N. A., Taylor-Blake B., Twomey M. A., Herrala A., Voikar V., Vihko P. (2008) Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron 60, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zimmermann H. (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 362, 299–309 [DOI] [PubMed] [Google Scholar]
- 19. Ohkubo S., Kimura J., Matsuoka I. (2000) Ecto-alkaline phosphatase in NG108–15 cells : a key enzyme mediating P1 antagonist-sensitive ATP response. Br. J. Pharmacol. 131, 1667–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Street S. E., Walsh P. L., Sowa N. A., Taylor-Blake B., Guillot T. S., Vihko P., Wightman R. M., Zylka M. J. (2011) PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol. Pain 7, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arcuino G., Lin J. H., Takano T., Liu C., Jiang L., Gao Q., Kang J., Nedergaard M. (2002) Intercellular calcium signaling mediated by point-source burst release of ATP. Proc. Natl. Acad. Sci. U.S.A. 99, 9840–9845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Halassa M. M., Florian C., Fellin T., Munoz J. R., Lee S. Y., Abel T., Haydon P. G., Frank M. G. (2009) Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang J., Kaasik K., Blackburn M. R., Lee C. C. (2006) Constant darkness is a circadian metabolic signal in mammals. Nature 439, 340–343 [DOI] [PubMed] [Google Scholar]
- 24. Dulla C. G., Dobelis P., Pearson T., Frenguelli B. G., Staley K. J., Masino S. A. (2005) Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron 48, 1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coward P., Chan S. D., Wada H. G., Humphries G. M., Conklin B. R. (1999) Chimeric G proteins allow a high throughput signaling assay of Gi-coupled receptors. Anal. Biochem. 270, 242–248 [DOI] [PubMed] [Google Scholar]
- 26. Zhang J. Y., Nawoschik S., Kowal D., Smith D., Spangler T., Ochalski R., Schechter L., Dunlop J. (2003) Characterization of the 5-HT6 receptor coupled to Ca2+ signaling using an enabling chimeric G protein. Eur. J. Pharmacol. 472, 33–38 [DOI] [PubMed] [Google Scholar]
- 27. Cooper J., Hill S. J., Alexander S. P. (1997) An endogenous A2B adenosine receptor coupled to cyclic AMP generation in human embryonic kidney (HEK 293) cells. Br. J. Pharmacol. 122, 546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naito Y., Lowenstein J. M. (1985) 5′-Nucleotidase from rat heart membranes: inhibition by adenine nucleotides and related compounds. Biochem. J. 226, 645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gerwins P., Nordstedt C., Fredholm B. B. (1990) Characterization of adenosine A1 receptors in intact DDT1 MF-2 smooth muscle cells. Mol. Pharmacol. 38, 660–666 [PubMed] [Google Scholar]
- 30. Schachter J. B., Sromek S. M., Nicholas R. A., Harden T. K. (1997) HEK293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology 36, 1181–1187 [DOI] [PubMed] [Google Scholar]
- 31. Nakata H., Yoshioka K., Kamiya T., Tsuga H., Oyanagi K. (2005) Functions of heteromeric association between adenosine and P2Y receptors. J. Mol. Neurosci. 26, 233–238 [DOI] [PubMed] [Google Scholar]
- 32. Yoshioka K., Saitoh O., Nakata H. (2001) Heteromeric association creates a P2Y-like adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 98, 7617–7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suzuki T., Namba K., Tsuga H., Nakata H. (2006) Regulation of pharmacology by hetero-oligomerization between A1 adenosine receptor and P2Y2 receptor. Biochem. Biophys. Res. Commun. 351, 559–565 [DOI] [PubMed] [Google Scholar]
- 34. Boyer J. L., Siddiqi S., Fischer B., Romero-Avila T., Jacobson K. A., Harden T. K. (1996) Identification of potent P2Y-purinoceptor agonists that are derivatives of adenosine 5′-monophosphate. Br. J. Pharmacol. 118, 1959–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu F., Wu H., Katritch V., Han G. W., Jacobson K. A., Gao Z. G., Cherezov V., Stevens R. C. (2011) Structure of an agonist-bound human A2A adenosine receptor. Science 332, 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lebon G., Warne T., Edwards P. C., Bennett K., Langmead C. J., Leslie A. G., Tate C. G. (2011) Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 474, 521–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olah M. E., Ren H., Ostrowski J., Jacobson K. A., Stiles G. L. (1992) Cloning, expression, and characterization of the unique bovine A1 adenosine receptor: studies on the ligand binding site by site-directed mutagenesis. J. Biol. Chem. 267, 10764–10770 [PMC free article] [PubMed] [Google Scholar]
- 38. Schenone S., Brullo C., Musumeci F., Bruno O., Botta M. (2010) A1 receptors ligands: past, present, and future trends. Curr. Top. Med. Chem. 10, 878–901 [DOI] [PubMed] [Google Scholar]
- 39. Fredholm B. B., IJzerman A. P., Jacobson K. A., Klotz K. N., Linden J. (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 53, 527–552 [PMC free article] [PubMed] [Google Scholar]
- 40. Elzein E., Zablocki J. (2008) A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin. Investig. Drugs 17, 1901–1910 [DOI] [PubMed] [Google Scholar]
- 41. Ashton T. D., Baker S. P., Hutchinson S. A., Scammells P. J. (2008) N6-substituted C5′-modified adenosines as A1 adenosine receptor agonists. Bioorg. Med. Chem. 16, 1861–1873 [DOI] [PubMed] [Google Scholar]
- 42. Cappellacci L., Franchetti P., Vita P., Petrelli R., Lavecchia A., Costa B., Spinetti F., Martini C., Klotz K. N., Grifantini M. (2008) 5′-Carbamoyl derivatives of 2′-C-methyl-purine nucleosides as selective A1 adenosine receptor agonists: affinity, efficacy, and selectivity for A1 receptor from different species. Bioorg. Med. Chem. 16, 336–353 [DOI] [PubMed] [Google Scholar]
- 43. Dalpiaz A., Scatturin A., Menegatti E., Bortolotti F., Pavan B., Biondi C., Durini E., Manfredini S. (2001) Synthesis and study of 5′-ester prodrugs of N6-cyclopentyladenosine, a selective A1 receptor agonist. Pharm. Res. 18, 531–536 [DOI] [PubMed] [Google Scholar]
- 44. Maillard M. C., Nikodijevi O., LaNoue K. F., Berkich D., Ji X. D., Bartus R., Jacobson K. A. (1994) Adenosine receptor prodrugs: synthesis and biological activity of derivatives of potent, A1-selective agonists. J. Pharm. Sci. 83, 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee K. S., Schubert P., Emmert H., Kreutzberg G. W. (1981) Effect of adenosine versus adenine nucleotides on evoked potentials in a rat hippocampal slice preparation. Neurosci. Lett. 23, 309–314 [DOI] [PubMed] [Google Scholar]
- 46. Pirotton S., Boeynaems J. M. (1993) Evidence that ATP, ADP, and AMP are not ligands of the striatal adenosine A2A receptors. Eur. J. Pharmacol. 241, 55–61 [DOI] [PubMed] [Google Scholar]
- 47. Zhou S. Y., Mamdani M., Qanud K., Shen J. B., Pappano A. J., Kumar T. S., Jacobson K. A., Hintze T., Recchia F. A., Liang B. T. (2010) Treatment of heart failure by a methanocarba derivative of adenosine monophosphate: implication for a role of cardiac purinergic P2X receptors. J. Pharmacol. Exp. Ther. 333, 920–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kumar T. S., Zhou S. Y., Joshi B. V., Balasubramanian R., Yang T., Liang B. T., Jacobson K. A. (2010) Structure-activity relationship of (N)-methanocarba phosphonate analogues of 5′-AMP as cardioprotective agents acting through a cardiac P2X receptor. J. Med. Chem. 53, 2562–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. El-Tayeb A., Iqbal J., Behrenswerth A., Romio M., Schneider M., Zimmermann H., Schrader J., Müller C. E. (2009) Nucleoside-5′-monophosphates as prodrugs of adenosine A2A receptor agonists activated by ecto-5′-nucleotidase. J. Med. Chem. 52, 7669–7677 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.