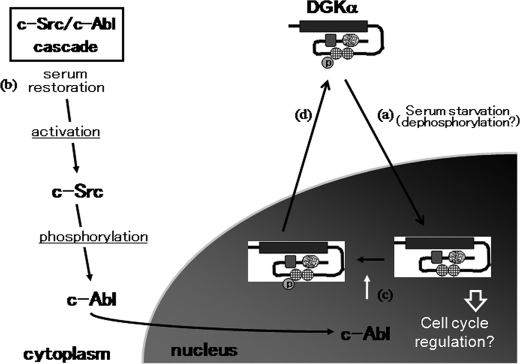
FIGURE 7.
Proposed model of DGKα nucleo-cytoplasmic shuttling. The C1 domain, the putative nuclear transportation signal, is blocked by the N-terminal region under normal conditions. Under serum starvation, DGKα is transported to the nucleus because the C1 domain becomes exposed (a). The trigger for the conformational change is unknown, but dephosphorylation may be a key step. This nuclear transportation is independent of DGKα activity. Serum restoration induces the sequential activation of c-Src and c-Abl (b). The activated c-Abl is transported to the nucleus and phosphorylates DGKα at residue 218 (c). The tyrosine phosphorylation triggers the nuclear export of DGKα, probably by inducing a conformational change regulated by the balance of the kinase domain and the N-terminal region as a nuclear export signal (d).