Abstract
Background
The 5-year survival rate for stage I non–small-cell lung cancer (NSCLC) of 50% to 70% indicates that our current staging methods do not adequately predict outcome. Empty spiracles homeobox 2 (EMX2) is a homeo–domain-containing transcription factor that regulates a key developmental pathway known to promote lung tumorigenesis. This study assessed the significance of EMX2 as a prognostic biomarker in lung adenocarcinoma including bronchioloalveolar carcinoma (BAC).
Patients and Methods
144 patients with lung adenocarcinoma undergoing surgical resection were studied. Quantitative real-time reverse transcriptase polymerase chain reaction and Immunohistochemistry were used to analyze EMX2 mRNA and protein expression, respectively. Association of EMX2 mRNA expression levels with clinical outcomes was evaluated using the Kaplan-Meier method and a multivariate Cox proportional hazards regression model.
Results
EMX2 mRNA expression was significantly downregulated in lung adenocarcinoma compared with matched adjacent normal tissue (P < .001). EMX2 protein expression was similarly found to be downregulated in lung adenocarcinoma. The EMX2-high mRNA expressing group had statistically significant better overall survival (OS) than the EMX2-low mRNA expressing group (P = .005). Subgroup analysis also demonstrated improved survival in stage I patients (P = .01) and patients with BAC (P = .03). Lastly, the EMX2-high mRNA expressing group had statistically significant better recurrence-free survival (RFS) than the EMX2-low mRNA expression group in patients with adenocarcinoma (P < .001).
Conclusion
EMX2 expression is downregulated in lung adenocarcinoma. Low EMX2 mRNA expression is significantly associated with decreased OS and RFS in patients with lung adenocarcinoma, particularly with stage I disease and BAC.
Keywords: Biomarker, Bronchioloalveolar carcinoma, Immunohistochemistry, Prognosis, RT-PCR
Introduction
Lung cancer is the most common cancer worldwide and the leading cause of cancer-related mortality.1 Adenocarcinoma is the most prevalent histologic subtype of lung cancer,2 comprising 35.3% and 73% of non–small-cell lung cancer (NSCLC) in the United States and Japan respectively.3,4 The molecular carcinogenesis of lung cancer is characterized by multiple alterations of gene expression and function. These alterations arise from a series of molecular and morphologic events that activate oncogenes such as K-ras and epidermal growth factor receptor (EGFR) and inactivate tumor suppressor genes such as p53 and p16.5 Despite these advances in our understanding of lung tumor biology, we have made little effect on the overall survival rate of patients with NSCLC in the past 30 years. The 5-year overall survival rate has changed little from 13% in 1975 to 16% today.6 It is estimated that 35% to 50% of early stage I non–small-cell lung cancer patients relapse within 5 years,6,7 indicating that our current staging methods are not adequate in predicting outcome.
Current efforts to improve lung cancer staging are centered on prognostic and predictive biomarkers.8,9 Several groups have shown that genetic pathways active in embryological development are reactivated in lung oncogenesis and may serve as important prognostic markers.8 In particular, strong evidence exists that activation of Wnt pathway not only drives lung tumorigenesis,10–12 but is prognostic of patient outcome in lung adenocarcinoma.13
Recently, it has been shown that empty spiracles homeobox 2 (EMX2), a human homolog of the Drosophila empty spiracles gene (ems), may regulate the Wnt pathway during development. The homeobox gene family encodes transcription factors, regulating morphogenesis and cell differentiation during embryogenesis by activating or repressing the expression of target genes.14 EMX2 is a homeodomain-containing transcription factor that has several important functions during early development. Mice harboring homozygous EMX2 mutations exhibit small olfactory bulbs and cerebral hemispheres.14 EMX2 also affects adult neural stem cell proliferation by controlling the frequency of symmetric divisions.15 In mammals, EMX2 controls reproduction by regulating endometrial cell proliferation16 and loss of EMX2 leads to cortical dysplasia via ectopic Wnt-1 expression in the developing telecephalon.17
In addition to its important role in embryologic development, several recent studies suggest that EMX2 may be involved in human tumorigenesis. For example, EMX2 has an antiproliferative function in the endometrium and exhibits decreased expression in endometrial tumors.16,18 It has also been reported that EMX2 is rarely methylated in nonseminoma testicular cancers.19 More recently, we have demonstrated that epigenetic silencing of the EMX2 expression in lung cancer may be important for aberrant activation of the canonical Wnt signaling and consequent proliferation and metastasis of lung cancer cells.20 Because the Wnt pathway genes are prognostic in lung adenocarcinoma and EMX2 may regulate the expression of Wnt genes, we hypothesized that EMX2 itself may serve as an important prognostic biomarker in lung adenocarcinoma. In this study, we analyzed EMX2 expression levels in patients with lung adenocarcinoma. We demonstrate that EMX2 mRNA and protein expression is downregulated in lung adenocarcinoma and that EMX2 mRNA expression may serve as an important prognostic biomarker in patients with lung adenocarcinoma.
Patients and Methods
Patients
One hundred forty-four patients with stage I–IV lung adenocarcinoma undergoing surgical resection at the University of California, San Francisco (UCSF) from July 1999 to August 2006 were entered into the study (Table 1). Sixty-four of these 144 patients had both tumor as well as matched adjacent normal tissue banked at the time of surgical resection. Patients receiving neoadjuvant chemotherapy or radiation therapy were excluded from this study. Tumor-node-metastasis (TNM) staging was performed according to the International System for Staging Lung Cancer pathologic classification system.21 All patients underwent mediastinal lymph node staging either intraoperatively as part of the surgical resection or via mediastinoscopy. Sixty-three of the 144 patients (44%) had bronchioloalveolar carcinoma (BAC); 21 of these patients had pure BAC and 42 had adenocarcinoma with BAC features (Table 1). Information on clinical variables and patient follow-up was extracted from a prospectively-maintained patient database (median follow-up for all patients was 42.8 ± 2.1 months extending to May 10, 2007; all but 1 patient had at least 6 months of follow-up). The primary endpoint was overall survival (OS). Vital status and date of death was determined by querying the Social Security Death Index with the subject’s social security number (available online at Social Security Death Index: http://www.deathindexes.com/ssdi.html). Recurrence-free survival (RFS) was defined as the time from surgery until radiographic evidence of recurrent disease or time until the last documented physician follow-up visit in the absence of recurrent disease. Patients consented to tissue specimen collection prospectively, and the study was approved by the University of California, San Francisco Institutional Review Board (CHR# H8714-17773-10).
Table 1.
EMX2 mRNA Expression and Clinical Characteristics in Lung Adenocarcinoma Patients
| Characteristics | Adenocarcinoma and BAC (N = 144) | ||||
|---|---|---|---|---|---|
| Number | % | EMX2 Expression | P Value | ||
| Age (Years) | |||||
| Median | 67.6 ± 0.9 | – | – | – | – |
| Range | 41–91 | – | – | – | – |
| Whole | – | 144 | 100 | 21.36 ± 4.68 | – |
| Young (< 50) | – | 9 | 6.2 | 8.36 ± 6.13 | – |
| Middle (50–75) | – | 98 | 68.1 | 20.79 ± 5.66 | – |
| Old (> 75) | – | 37 | 25.7 | 26.04 ± 10.29 | NS |
| Sex | |||||
| Male | – | 54 | 37.5 | 15.34 ± 5.91 | – |
| Female | – | 90 | 62.5 | 24.97 ± 6.58 | P = .04 |
| Race | |||||
| Caucasian | – | 110 | 76.4 | 20.39 ± 5.43 | – |
| Asian | – | 22 | 15.3 | 20.42 ± 10.50 | – |
| Others | – | 12 | 8.3 | 31.04 ± 20.21 | NS |
| Smoking | |||||
| Never | – | 33 | 22.9 | 19.54 ± 8.20 | – |
| Smoker | – | 107 | 74.3 | 21.27 ± 5.73 | – |
| Past | – | 68 | 47.2 | 24.56 ± 8.22 | – |
| Current | – | 39 | 27.1 | 15.55 ± 6.50 | – |
| Unknown | – | 4 | 2.8 | 38.62 ± 21.72 | NS |
| Pack Per Year | |||||
| Median | 34.4 ± 3.0 | – | – | – | – |
| Range | 0–160 | – | – | – | – |
| Sex, Smoking | |||||
| Male, Smoker | – | 46 | 31.9 | 17.11 ± 6.91 | – |
| Male, Non-smoker | – | 8 | 5.6 | 5.18 ± 2.66 | – |
| Female, Smoker | – | 61 | 42.4 | 24.41 ± 8.63 | – |
| Female, Non-smoker | – | 25 | 17.3 | 24.14 ± 10.68 | – |
| Unknown | – | 4 | 2.8 | 38.62 ± 21.72 | NS |
| Tumor Size (cm) | |||||
| Median | 3.5 ± 0.2 | – | – | – | – |
| Range | 0.8–13.0 | – | – | – | – |
| 3 cm or less | – | 76 | 52.8 | 28.74 ± 8.02 | – |
| Over 3 cm | – | 68 | 47.2 | 13.24 ± 4.11 | NS |
| Pathologic Stage | |||||
| Stage I | – | 91 | 63.2 | 18.55 ± 4.29 | – |
| Stage II | – | 18 | 12.5 | 16.09 ± 11.19 | – |
| Stage III | – | 25 | 17.4 | 15.70 ± 8.57 | – |
| Stage IV | – | 10 | 6.9 | 72.00 ± 46.35 | NS |
| Histology | |||||
| Adenocarcinoma | – | 81 | 56.3 | 26.03 ± 7.56 | – |
| BAC | – | 63 | 43.7 | 15.35 ± 4.42 | – |
| Mixed BAC | – | 42 | 29.1 | 18.24 ± 6.34 | – |
| Pure BAC | – | 21 | 14.6 | 9.57 ± 3.83 | NS |
| ECOG PS | |||||
| 0 | – | 76 | 52.8 | 19.31 ± 4.90 | – |
| 1 | – | 29 | 20.1 | 11.18 ± 6.53 | – |
| 2 | – | 6 | 4.2 | 1.73 ± 0.70 | – |
| Unknown | – | 33 | 22.9 | 38.60 ± 15.80 | NS |
| Surgical Procedure | |||||
| Wedge Resection | – | 13 | 9.0 | 44.31 ± 33.91 | – |
| Segmentectomy | – | 5 | 3.5 | 15.17 ± 10.23 | – |
| Lobectomy | – | 113 | 78.5 | 19.74 ± 4.43 | – |
| Bilobectomy | – | 7 | 4.9 | 17.45 ± 14.72 | – |
| Pnumonectomy | – | 6 | 4.1 | 11.83 ± 11.07 | NS |
| Vital Status | |||||
| Alive | – | 91 | 63.2 | 30.51 ± 7.16 | – |
| Dead | – | 53 | 36.8 | 5.64 ± 1.91 | P = .003 |
| Recurrence | |||||
| Positive | – | 55 | 38.2 | 6.48 ± 2.11 | – |
| Negative | – | 74 | 51.4 | 22.75 ± 5.46 | P = .007 |
| Follow-up (Months) | |||||
| Median | 41.6 ± 2.3 | – | – | – | – |
| Range | 0.3–117.3 | – | – | – | – |
Abbreviations: BAC = bronchioloalveolar carcinoma; ECOG PS = Eastern Cooperative Oncology Group performance status; EMX2 = empty spiracles homeobox 2; NS = not significant.
Tissue RNA Extraction and Quantitative Real-Time Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR)
Fresh samples were collected from patients at the time of surgical resection and were promptly snap-frozen in liquid nitrogen and stored at −170°C in the UCSF Thoracic Oncology Tissue Bank. Tissues were macrodissected as 1 cm3 blocks by 1 of the investigators of the study and the primary tumor and adjacent normal tissue specimens were examined microscopically by a pathologist. In previous validation, 95% of these macrodissected tissue specimens yield tumor tissue sections with greater than 70% tumor cells. Total RNA was extracted from fresh-frozen tissue specimens using the TRIzol LS method (Invitrogen). cDNA synthesis and quantitative Taqman PCR were performed as previously described.22 All RNA extracts were analyzed by Nanodrop spectrophotometry for concentration and purity using a Nanodrop ND-1000 (ThermoScientific). Any samples with a 260/280 or 260/230 ratio of less than 2.0 were reextracted. TaqMan Real-Time PCR primers and probes for EMX2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems. EMX2 and GAPDH mRNA expression were assayed in triplicate using the ABI 7300 Real-time PCR System using 200 ng input RNA. For relative EMX2 mRNA expression between tumor and matched normal samples, relative gene expression is represented as 2−deltaCt ×1000. For survival analysis, all tumor samples were compared with commercially prepared total RNA from adult normal lung tissue (BioChain, Hayward, CA) and normalized using GAPDH as an internal control gene. Relative EMX2 mRNA expression values were calculated using the 2ΔΔCt comparative CT method.23
Immunohistochemistry
Immunohistochemistry (IHC) staining of EMX2 was performed using a rabbit anti-EMX2 polyclonal antibody (Pierce Scientific) at 1:500 dilution. Five-micron tissue slides from tumor and adjacent normal lung tissue were de-paraffinized using xylene. Heat-mediated antigen retrieval was performed using citrate buffer (BioGenex Laboratories, San Ramon, CA). Antibody staining was visualized with DAB (Histostain Plus Broad Spectrum, Invitrogen) and hematoxylin counterstain (Fisher Scientific). Representative 40× fields were examined for positive nuclear staining and the mean number of positive cells in each tumor and matched normal tissue sample was compared using a 2-tailed paired t test.
Statistical Analysis
Mean relative mRNA expression between matched tumor and normal samples was compared using a 2-tailed paired t test. The Kaplan-Meier method was used to estimate OS and RFS. Differences in survival between the low-risk group (high EMX2 mRNA expression) and high-risk group (low EMX2 mRNA expression) were analyzed by log-rank test. Optimal cut off points for partitioning into high/low risk survival subgroups based on threshold EMX2 mRNA expression at the cut off point were determined using survival trees. Assessment of the predictive reproducibility of the partition made recourse to cross-validation.24 A multivariate Cox proportional hazards regression model was used to assess the association between all clinicopathological varibles (age, sex, race, smoking status, tumor size, pathologic stage, histology, Eastern Cooperative Oncology Group performance status [ECOG PS], and procedure), EMX2 mRNA expression, and survival. The associations between gene expression and discrete clinical factors were analyzed by the t test, analysis of variance (ANOVA) with Bonferroni/Dunn test, Mann-Whitney U test for variables with 2 categories, and the Kruskal-Wallis test for variables with more than 2 categories. All reported P values were 2-sided. A P value of .05 or less was considered to be significant.
Results
EMX2 Expression Is Downregulated in Lung Adenocarcinoma
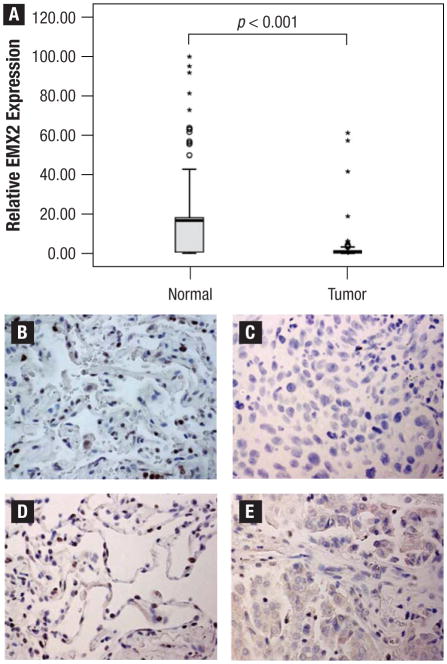
We first examined EMX2 mRNA expression in 64 patients who had paired tumor as well as matched adjacent normal tissue banked at the time of surgical resection. Forty-six of 64 (71.8%) lung adenocarcinoma specimens had less EMX2 mRNA expression than their matched adjacent normal tissues (Figure 1A). The mean values of relative EMX2 mRNA expression as assessed by real-time RT-PCR were 3.78 and 18.01 in tumor specimens and their matched adjacent normal tissues, respectively. This difference was statistically significant (P < .001). We also examined EMX2 protein expression by IHC in 20 separate lung adenocar-cinoma specimens. EMX2 protein downregulation in tumor tissues when compared with their matched normal tissues (Figure 1B versus 1C; Figure 1D versus 1E) was observed, with an average of 28.7 versus 8.0 positively-stained cells per 40× field respectively (P < .001), consistent with our real-time RT-PCR results.
Figure 1.
Empty Spiracles Homeobox 2 (EMX2) Expression Is Downregulated in Lung Adenocarcinoma.(A) Quantitative Reverse-transcriptase Polymerase Chain Reaction (RT-PCR) Result of 64 Tumors and Their Matched Normal Lungs is Shown. Twenty-fifth and 75th Percentiles are Represented as Box Margins, 10th and 90th Percentiles are Represented as Error Bars, and the Median is Represented as a Line in the Box. Outlying Data are Represented as Dots (90%–95% Percentile) and Stars (Greater Than 95% Percentile). (B) and (C); (D) and (E): Immunohistochemistry (IHC) Examples of EMX2 Protein in Matched Normal Lung Tissues ((B) and (D)) and Tumor Tissues ((C) and (E)) From 2 Adenocarcinoma Patients. EMX2 Protein is Represented by Positive Nuclear Staining. Pictures were Taken Under Using a Light Microscope (Magnification 40×)
EMX2 mRNA Expression Is Associated With Improved Survival in Lung Adenocarcinoma
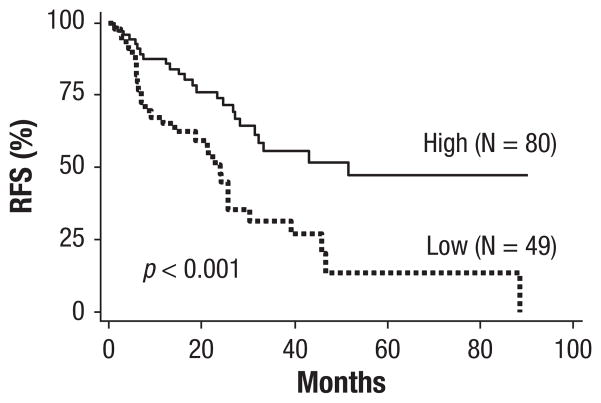
EMX2 mRNA expression was assessed on 144 frozen lung adenocarcinoma specimens by real-time PCR. By univariate analysis, we found that EMX2 mRNA expression was associated with gender (P = .04) (Table 1). There was no significant association between EMX2 mRNA expression and tumor stage, age, smoking status, histology, surgical procedure, or ECOG PS (Table 1). EMX2 mRNA expression was associated with improved overall survival (P = .003) and decreased recurrence (P = .007) (Table 1). When examining all clinical variables as well as EMX2 mRNA expression by Cox multivariate regression analysis, high EMX2 mRNA expression (hazard ratio 0.44; CI, 0.23–0.85; P = .02) and pathologic stages I, III, and IV were significantly associated with overall survival (Table 2). Kaplan-Meier analysis using Cox proportional hazards modeling also demonstrated strong associations between EMX2 mRNA expression and survival. Overall survival was significantly better in patients in the EMX2-high expressing group compared with those in the EMX2-low expressing group in all patients (P = .005; the median survival: high [not reached] versus low [60 months]) (Figure 2A). This association was also examined using subgroup analysis by stage. In 91 stage I patients, overall survival was significantly better in EMX2-high expressing patients using 2 different cut off points: 1.29 (P = .01; not reached versus 56 months) (Figure 2B) and 6.77 (P = .006; not reached versus 98 months) (Figure 2C). A statistically significant difference in survival was not observed in patients with stages II to IV (P = .36) (Figure 2D). Kaplan-Meier analysis also demonstrated an association between EMX2 mRNA expression and overall survival by histologic subtype. A significant difference in overall survival was seen in 63 patients with BAC (P = .03; not reached versus 56 months) (Figure 2E), but not in 81 patients with pure adenocarcinoma (P = .13) (Figure 2F). Lastly, there was a significant difference in recurrence-free survival in 129 patients for whom follow-up recurrence data were available. The median recurrence-free survival in EMX2-low expressing group was 24 months, compared with 52 months for the EMX2-high expressing group (P < .001) (Figure 3).
Table 2.
Cox Multivariate Model in Lung Adenocarcinoma and BAC Patients
| Variable | Hazard Ratio | (95% CI) | P Value |
|---|---|---|---|
| EMX2 Expression | |||
| High-expression group | 0.44 | 0.23–0.85 | .02 |
| Pathologic stage (compared with stage I) | 0.81 | 0.71–1.04 | .03 |
| Stage II | 1.36 | 0.50–3.72 | .55 |
| Stage III | 2.47 | 1.13–5.41 | .02 |
| Stage IV | 3.88 | 1.41–10.69 | .009 |
| Histology | |||
| BAC (compared with adenocarcinoma) | 0.60 | 0.80–4.90 | .12 |
Abbreviations: BAC = bronchioloalveolar carcinoma; EMX2 = empty spiracles homeobox 2.
Figure 2.
Kaplan-Meier Estimates of Overall Survival (OS) Associated with Empty Spiracles Homeobox 2 (EMX2) mRNA Expression. (A) OS in 144 Patients with Adenocarcinoma; OS in 91 Stage I Patients Using (B) 1.29 and (C) 6.77 as Cut-off Points; (D) OS in 53 Stage II–IV Patients; (E) OS in 63 Patients with Bronchioloalveolar Carcinoma (BAC); and (F) OS in 81 Pure Adenocarcinoma Patients
Figure 3.
Kaplan-Meier Estimates of Recurrence-Free Survival Associated With Empty Spiracles Homeobox 2 (EMX2) mRNA Expression. Quantitative Reverse-transcriptase Polymerase Chain Reaction (RT-PCR) in 129 Adenocarcinoma Patients
Discussion
Our data demonstrate that EMX2 is downregulated in lung adenocarcinoma and acts as a prognostic marker of overall and recurrence-free survival. Cross-validation was used for both model selection as well as validation. Cross-validation is operationally equivalent to having a training and validation cohort but uses data more efficiently by using repeated data-splitting.25 Because cross-validation avoids model over-fitting and generates more accurate estimates of cut off points,25 it is widely employed to generate prognostic marker models.22,26–30
EMX2 mRNA expression was associated with improved overall survival in stage I patients. This is consistent with previously published studies in which prognostic markers are better predictors of survival in early-stage versus late stage NSCLC.22,31 EMX2 mRNA expression was also associated with improved overall survival in patients with adenocarcinoma with BAC. Bronchioloalveolar carcinoma is a subset of adenocarcinoma that is characterized by noninvasive growth along alveolar septae.32 It is more prevalent in women, nonsmokers, and Asians.32 Despite the lack of stromal, vascular, or pleural invasion, BAC is malignant and surgical resection is currently the mainstay of curative treatment.32 To date, although several prognostic markers for adenocarcinoma and NSCLC exist,8,9 few are specific to BAC. Saad et al have demonstrated that protein expression of HER2/neu is associated with increased mortality in 32 patients with BAC nonmucinous type33 and thyroid transcription factor-1 immunohistochemical expression exhibits a trend toward increased survival in 50 patients with BAC.34 Gao et al demonstrated that glutathione S-transferase pi 1 methylation and DNA (cytosine-5)-methyltransferase 1 protein expression were significantly correlated with increased recurrence and decreased overall survival in 36 patients with BAC.35 Lastly, Sarkaria et al demonstrated that low-grade squamous cell carcinoma–related oncogene protein expression in tumor-adjacent benign cores was correlated with decreased overall survival in 118 patients with BAC.36 In contrast to these studies, we have identified a prognostic molecular marker that may be measured by gene expression analysis in the largest reported cohort of patients with BAC.
The biologic mechanisms underlying the functional role of EMX2 as a prognostic marker in lung adenocarcinoma biology are still unclear. This is partly because more studies are needed which specifically examine the role of EMX2 in lung tumorigenesis. Because loss of EMX2 is associated with increased ectopic Wnt-1 expression17 and activation of the Wnt pathway has been demonstrated to be oncogenic in NSCLC,10–12 it is tempting to speculate that decreased EMX2 expression acts as a marker for increased Wnt pathway activation during lung oncogenesis. Because Wnt pathway members have been shown to be prognostic markers in lung adenocarcinoma,13,22 this would illuminate the functional mechanism of EMX2 as a prognostic marker. This hypothesis, indeed, is supported by our recent evidence that methylation silencing of EMX2 is associated with aberrant activation of the canonical Wnt signaling in lung cancer and that EMX2 suppresses proliferation and metastasis of lung cancer cells.20
This study has several limitations. First, it uses banked frozen tissue specimens. Although the use of such specimens in studies of prognostic markers in NSCLC is widespread, using frozen tissue specimens for analysis in the clinical setting is highly impractical.9 Formalin-fixed paraffin-embedded tissue specimens are ubiquitous, stable at room temperature, and easy to handle. Extraction of RNA from formalin-fixed paraffin-embedded tissues and gene expression studies on this RNA using real-time PCR is now possible.37 Thus, validation of this study should be performed in a large cohort of formalin-fixed paraffin-embedded lung adenocarcinoma samples and is currently underway in our laboratory. Secondly, as is typical of frozen tissue samples banked prospectively for genomic analysis, the actual pieces of tumor and normal adjacent tissue used in this study were macrodissected by 1 of the investigators in this study and not directly examined by a pathologist. Nevertheless, the primary tumor and adjacent normal tissue specimens were examined microscopically by a pathologist, a method which has been shown to have high correlation in previously published work.22 In addition, previous validation has demonstrated that 95% of macrodissected banked tissue yields tumor tissue sections with greater than 70% tumor cells.22 Lastly, if greater than 30% of normal tissue was inadvertently included as part of the tumor sample, one would expect this to bias our results toward the null hypothesis of no association between EMX2 mRNA expression and survival.
Despite these limitations, our data demonstrate the prognostic significance of EMX2 mRNA expression in lung adenocarcinoma and BAC. The importance of finding reliable prognostic markers for NSCLC, especially early-stage NSCLC, is becoming increasingly apparent. The 5-year survival rate for stage I NSCLC is 50% to 70%,38 indicating that our current staging methods do not adequately stratify outcome. As a result, the current gold standard of treating early-stage lung cancer by lobectomy and mediastinal lymph node dissection may represent overtreatment in patients with early-stage tumors expressing biomarkers associated with good outcomes. At the same time, because neoadjuvant and adjuvant therapy is not considered standard of care for early-stage lung cancer, we may be undertreating patients with early-stage tumors expressing biomarkers associated with poor outcomes by surgical resection alone.
Other biomarkers have been demonstrated to have prognostic significance in NSCLC using immunohistochemical, immunofluorescence, methylation, and mutation analysis.8 However, the discovery of EMX2 as a novel molecular prognostic marker that can be assessed by gene expression using real time RT-PCR is significant because such biomarkers may offer more direct and immediate translation into clinical practice. Although the prognostic significance of this and other real-time RT-PCR-based molecular markers needs to be validated in formalin-fixed paraffin embedded tissue specimens, the feasibility and utility of a prognostic assay using molecular markers assessed by real-time RT-PCR in formalin-fixed paraffin embedded tissue specimens has already been demonstrated the field of breast oncology.39 It is hoped that EMX2 mRNA expression may be incorporated into a similar practical assay for lung oncology. Making such an assay available to patients with BAC may become particularly important as the incidence of BAC in patients with NSCLC seems to be rising32 in conjunction with the yearly decline of cigarette smoking in the United States.40
Conclusion
Our data demonstrate that the homeo-domain containing transcription factor EMX2 is downregulated in lung adenocarcinoma. Low EMX2 mRNA expression is associated with decreased disease-free survival and overall survival in lung adenocarcinoma patients, particularly in patients with stage I disease and BAC. Defining the relationship between EMX2 expression and clinical prognosis will enhance our understanding of lung cancer biology and be of help to improve risk stratification and therapeutic options for lung adenoarcinoma patients. The discovery of this novel putative prognostic marker that can be assessed by gene expression using real-time PCR may also offer more direct translation into clinical practice, either alone or in combination with other biomarkers.
Acknowledgments
This work was supported by Joan’s Legacy: Uniting Against Lung Cancer Research Grant, NIH/NCI R01CA125030, and the Eileen D. Ludwig Endowed for Thoracic Oncology Research (to B. He); The Bonnie J. Addario Lung Cancer Foundation, the Kazan, McClain, Abrams, Fernandez, Lyons, Greenwood, Harley & Oberman Foundation, and the Barbara Isackson Lung Cancer Research Fund (to D. Jablons).
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Nordquist LT, Simon GR, Cantor A, et al. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest. 2004;126:347–51. doi: 10.1378/chest.126.2.347. [DOI] [PubMed] [Google Scholar]
- 3.Little AG, Gay EG, Gaspar LE, et al. National survey of non-small cell lung cancer in the United States: epidemiology, pathology and patterns of care. Lung Cancer. 2007;57:253–60. doi: 10.1016/j.lungcan.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Sekine I, Nishiwaki Y, Yokose T, et al. Young lung cancer patients in Japan: different characteristics between the sexes. Ann Thorac Surg. 1999;67:1451–5. doi: 10.1016/s0003-4975(99)00171-x. [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 7.Guo L, Ma Y, Ward R, et al. Constructing molecular classifiers for the accurate prognosis of lung adenocarcinoma. Clin Cancer Res. 2006;12:3344–54. doi: 10.1158/1078-0432.CCR-05-2336. [DOI] [PubMed] [Google Scholar]
- 8.Coate LE, John T, Tsao MS, et al. Molecular predictive and prognostic markers in non-small-cell lung cancer. Lancet Oncol. 2009;10:1001–10. doi: 10.1016/S1470-2045(09)70155-X. [DOI] [PubMed] [Google Scholar]
- 9.Kratz JR, Jablons DM. Genomic prognostic models in early-stage lung cancer. Clin Lung Cancer. 2009;10:151–7. doi: 10.3816/CLC.2009.n.021. [DOI] [PubMed] [Google Scholar]
- 10.He B, Barg RN, You L, et al. Wnt signaling in stem cells and non-small-cell lung cancer. Clin Lung Cancer. 2005;7:54–60. doi: 10.3816/CLC.2005.n.022. [DOI] [PubMed] [Google Scholar]
- 11.Mazieres J, He B, You L, et al. Wnt signaling in lung cancer. Cancer Lett. 2005;222:1–10. doi: 10.1016/j.canlet.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 12.Uematsu K, He B, You L, et al. Activation of the Wnt pathway in non small cell lung cancer: evidence of dishevelled overexpression. Oncogene. 2003;22:7218–21. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen DX, Chiang AC, Zhang XH, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalton D, Chadwick R, McGinnis W. Expression and embryonic function of empty spiracles: a Drosophila homeo box gene with two patterning functions on the anterior-posterior axis of the embryo. Genes Dev. 1989;3:1940–56. doi: 10.1101/gad.3.12a.1940. [DOI] [PubMed] [Google Scholar]
- 15.Galli R, Fiocco R, De Filippis L, et al. Emx2 regulates the proliferation of stem cells of the adult mammalian central nervous system. Development. 2002;129:1633–44. doi: 10.1242/dev.129.7.1633. [DOI] [PubMed] [Google Scholar]
- 16.Taylor HS, Fei X. Emx2 regulates mammalian reproduction by altering endometrial cell proliferation. Mol Endocrinol. 2005;19:2839–46. doi: 10.1210/me.2005-0130. [DOI] [PubMed] [Google Scholar]
- 17.Ligon KL, Echelard Y, Assimacopoulos S, et al. Loss of Emx2 function leads to ectopic expression of Wnt1 in the developing telencephalon and cortical dysplasia. Development. 2003;130:2275–87. doi: 10.1242/dev.00421. [DOI] [PubMed] [Google Scholar]
- 18.Noonan FC, Mutch DG, Ann Mallon M, et al. Characterization of the homeodomain gene EMX2: sequence conservation, expression analysis, and a search for mutations in endometrial cancers. Genomics. 2001;76:37–44. doi: 10.1006/geno.2001.6590. [DOI] [PubMed] [Google Scholar]
- 19.Lind GE, Skotheim RI, Fraga MF, et al. Novel epigenetically deregulated genes in testicular cancer include homeobox genes and SCGB3A1 (HIN-1) J Pathol. 2006;210:441–9. doi: 10.1002/path.2064. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto J, Hirata T, Chen Z, et al. EMX2 is epigenetically silenced and suppresses growth in human lung cancer [published erratum appears in: Oncogene 2010; 29:5976. Beltran, A (corrected to Yagui-Beltran, A)] Oncogene. 2010;29:5969–75. doi: 10.1038/onc.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–7. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 22.Raz DJ, Ray MR, Kim JY, et al. A multigene assay is prognostic of survival in patients with early-stage lung adenocarcinoma. Clin Cancer Res. 2008;14:5565–70. doi: 10.1158/1078-0432.CCR-08-0544. [DOI] [PubMed] [Google Scholar]
- 23.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 24.Therneau TM, Atkinson EJ. Technical Report. Mayo Foundation; Rochester, MN: 1997. An introduction to recursive partitioning using the RPART routines. [Google Scholar]
- 25.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–24. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 27.Bianchi F, Nuciforo P, Vecchi M, et al. Survival prediction of stage I lung adenocarcinomas by expression of 10 genes. J Clin Invest. 2007;117:3436–44. doi: 10.1172/JCI32007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potti A. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. N Engl J Med. 2006;355:570–80. doi: 10.1056/NEJMoa060467. [DOI] [PubMed] [Google Scholar]
- 29.Raponi M, Zhang Y, Yu J, et al. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res. 2006;66:7466–72. doi: 10.1158/0008-5472.CAN-06-1191. [DOI] [PubMed] [Google Scholar]
- 30.Reed CE, Graham A, Hoda RS, et al. A simple two-gene prognostic model for adenocarcinoma of the lung. J Thorac Cardiovasc Surg. 2008;135:627–34. doi: 10.1016/j.jtcvs.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen HY, Yu SL, Chen CH, et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med. 2007;356:11–20. doi: 10.1056/NEJMoa060096. [DOI] [PubMed] [Google Scholar]
- 32.Raz DJ, He B, Rosell R, et al. Bronchioloalveolar carcinoma: a review. Clin Lung Cancer. 2006;7:313–22. doi: 10.3816/CLC.2006.n.012. [DOI] [PubMed] [Google Scholar]
- 33.Saad RS, Liu Y, Han H, et al. Prognostic significance of HER2/neu, p53, and vascular endothelial growth factor expression in early stage conventional adenocarcinoma and bronchioloalveolar carcinoma of the lung. Mod Pathol. 2004;17:1235–42. doi: 10.1038/modpathol.3800171. [DOI] [PubMed] [Google Scholar]
- 34.Saad RS, Liu YL, Han H, et al. Prognostic significance of thyroid transcription factor-1 expression in both early-stage conventional adenocarcinoma and bronchi-oloalveolar carcinoma of the lung. Hum Pathol. 2004;35:3–7. doi: 10.1016/j.humpath.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Gao P, Yang X, Xue YW, et al. Promoter methylation of glutathione S-transferase pi1 and multidrug resistance gene 1 in bronchioloalveolar carcinoma and its correlation with DNA methyltransferase 1 expression. Cancer. 2009;115:3222–32. doi: 10.1002/cncr.24369. [DOI] [PubMed] [Google Scholar]
- 36.Sarkaria IS, Pham D, Ghossein RA, et al. SCCRO expression correlates with invasive progression in bronchioloalveolar carcinoma. Ann Thorac Surg. 2004;78:1734–41. doi: 10.1016/j.athoracsur.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 37.von Ahlfen S, Missel A, Bendrat K, et al. Determinants of RNA quality from FFPE samples. PLoS One. 2007;2:e1261. doi: 10.1371/journal.pone.0001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–71. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 39.Paik S. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. Oncologist. 2007;12:631–5. doi: 10.1634/theoncologist.12-6-631. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults–United States, 2007 [published erratum appears in: MMWR Morb Mortal Wkly Rep 2008; 57:1281] MMWR Morb Mortal Wkly Rep. 2008;57:1221–6. [PubMed] [Google Scholar]