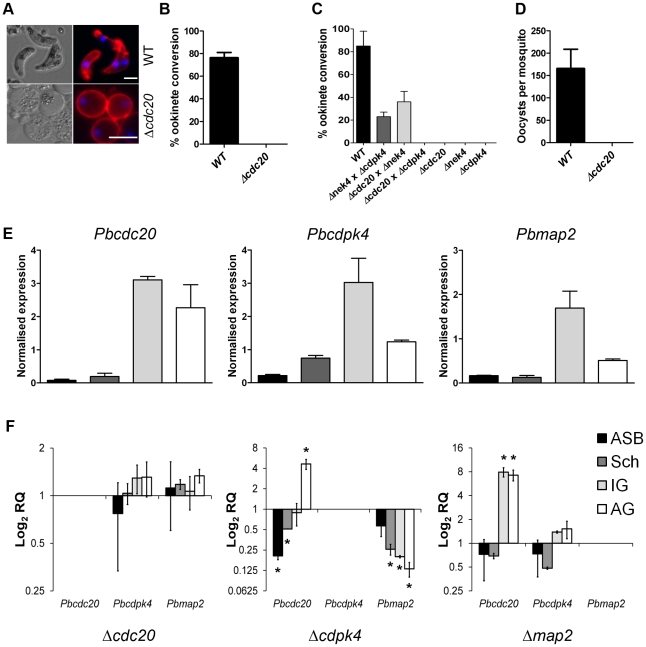
Figure 3. Phenotypic analysis of Δcdc20.
A. Immunofluorescence images of Plasmodium cultures after 24 hr in vitro immunostained for the female gamete/zygote/ookinete marker P28 (red) and counterstained with the nuclear marker Hoechst (blue). Development of elongated ookinetes was completely ablated in Δcdc20 lines, which produced only round female gametes. Bar = 5 µm. B. Bar graph illustrating ookinete conversion in wild-type and Δcdc20 parasites. The conversion rate is the percentage of P28-positive parasites that had successfully differentiated into elongated ‘banana-shaped’ ookinetes (error bar = arithmetic mean ±SD; n = 3). C. Ookinete conversion after crossing Δcdc20 parasites with a female-defective nek4 mutant (Δnek4) and a male-defective cdpk4 mutant (Δcdpk4). Wild-type parasites were used as a control. Bar graph represents the percentage of round P28-positive parasites that had converted into elongated ookinetes (arithmetic mean ±SD; n = 3). D. Bar graph showing average numbers of oocysts per gut (error bar indicates ±SEM; n = 60 of wild-type or Δcdc20 infected mosquitoes from three independent experiments). Overall infection prevalence was 80% for wild-type and 0% for Δcdc20. E. Wild-type mRNA expression of cdc20, cdpk4 and map2 relative to hsp70 and arginyl-tRNA synthetase as endogenous controls (ΔΔCt method). Error bars represent ±SEM, n = 3 from three independent experiments. The key to the shading of bars is indicated in F. F. Relative expression of cdc20, cdpk4 and map2 in Δcdc20, Δcdpk4 and Δmap2 parasites compared to wild-type parasites (Pfaffl method). Error bars represent ±SEM, n = 3 from three independent experiments. ASB = Asexual blood; Sch = Schizont; IG = Inactivated gametocytes; AG = Activated gametocytes; RQ = relative quantification.