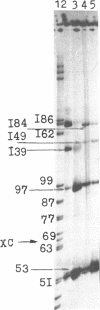
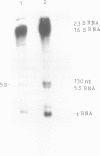
Abstract
An artificial gene comprising 183 base pairs has been assembled by template-directed condensation of 35- to 53-membered oligodeoxyribo nucleotides with cyanogen bromide as a condensing agent. The reaction is complete within several minutes at 0 degrees C in buffer. The resulting mini-gene was cloned and expressed in vivo and in vitro. We have also found that the polymerase inhibition technique (toe-printing) is a good way to ascertain that translation initiation complexes form in the case of single-stranded DNAs as well. Thus, along with the fully chemical assembly of synthetic genes, a rapid and sufficiently reliable method for determining their ribosome-binding properties was developed.
Full text
PDF
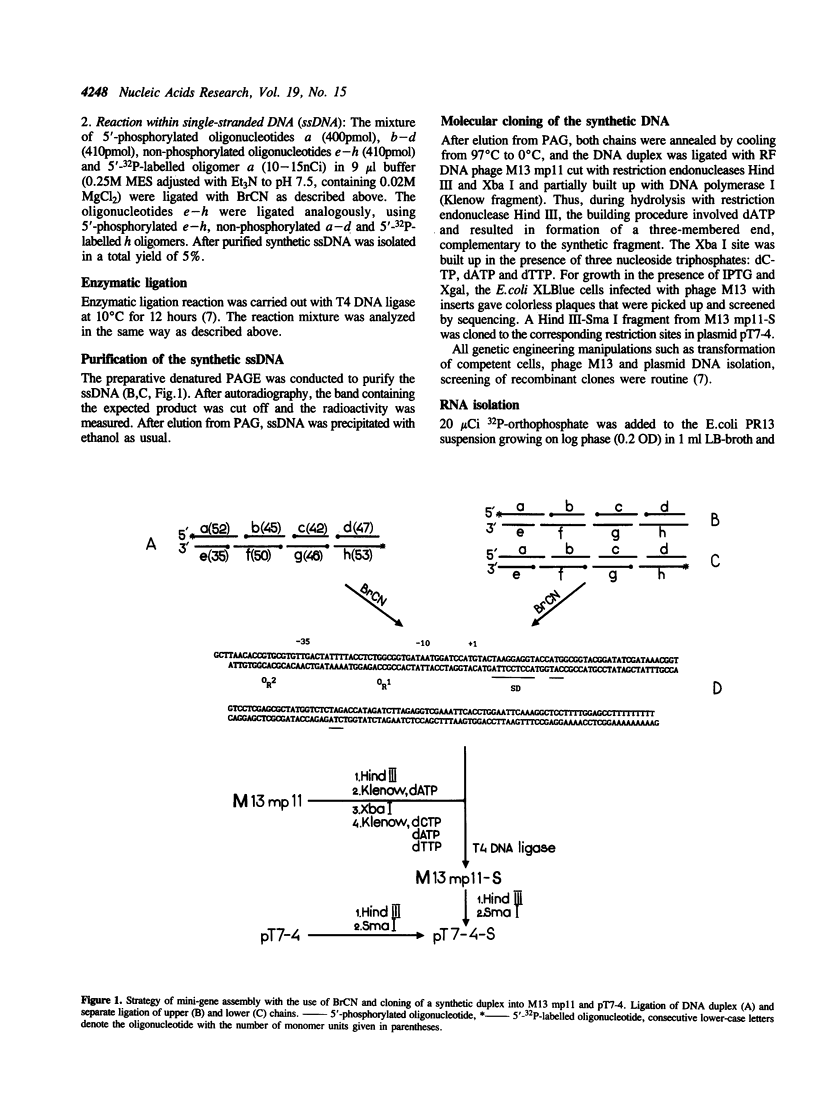



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Büchi H., Caruthers M. H., Gupta N., Khorana H. G., Kleppe K., Kumar A., Ohtsuka E., Rajbhandary U. L., Van de Sande J. H. Total synthesis of the gene for an alanine transfer ribonucleic acid from yeast. Nature. 1970 Jul 4;227(5253):27–34. doi: 10.1038/227027a0. [DOI] [PubMed] [Google Scholar]
- Balakin A. G., Skripkin E. A., Shatskii I. N., Van Duin Ia, Bogdanov A. A. Lokalizatsiia uchastkov mRNK, ékraniruemykh ribosomami v initsiatornykh kompleksakh, pri pomoshchi reaktsii obratnoi transkriptsii. Dokl Akad Nauk SSSR. 1989;305(4):1000–1003. [PubMed] [Google Scholar]
- Dolinnaya N. G., Sokolova N. I., Gryaznova O. I., Shabarova Z. A. Site-directed modification of DNA duplexes by chemical ligation. Nucleic Acids Res. 1988 May 11;16(9):3721–3738. doi: 10.1093/nar/16.9.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz D., McPheeters D. S., Traut R., Gold L. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- Jinks-Robertson S., Gourse R. L., Nomura M. Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell. 1983 Jul;33(3):865–876. doi: 10.1016/0092-8674(83)90029-6. [DOI] [PubMed] [Google Scholar]
- Kagramanova V. K., Derckacheva N. I., Mankin A. S. The complete nucleotide sequence of the arcaebacterial plasmid pHSB from Halobacterium, strain SB3. Nucleic Acids Res. 1988 May 11;16(9):4158–4158. doi: 10.1093/nar/16.9.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Traut R. R., Noller H., Pearson P., Delius H. Ribosomal proteins of Escherichia coli. II. Proteins from the 30 s subunit. J Mol Biol. 1968 Feb 14;31(3):441–461. doi: 10.1016/0022-2836(68)90420-8. [DOI] [PubMed] [Google Scholar]
- Shabarova Z. A. Chemical development in the design of oligonucleotide probes for binding to DNA and RNA. Biochimie. 1988 Oct;70(10):1323–1334. doi: 10.1016/0300-9084(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Shelness G. S., Williams D. L. Secondary structure analysis of apolipoprotein II mRNA using enzymatic probes and reverse transcriptase. Evaluation of primer extension for high resolution structure mapping of mRNA. J Biol Chem. 1985 Jul 15;260(14):8637–8646. [PubMed] [Google Scholar]
- Skripkin E. A., Bogdanova S. L., Kopylov A. M., Bogdanov A. A. Konstruirovanie i ékspressiia genov, kodiruiushchikh korotkie gibridnye mRNK Escherichia coli. Dokl Akad Nauk SSSR. 1986 Mar-Apr;287(1):237–241. [PubMed] [Google Scholar]
- Sokolova N. I., Ashirbekova D. T., Dolinnaya N. G., Shabarova Z. A. Chemical reactions within DNA duplexes. Cyanogen bromide as an effective oligodeoxyribonucleotide coupling agent. FEBS Lett. 1988 May 9;232(1):153–155. doi: 10.1016/0014-5793(88)80406-x. [DOI] [PubMed] [Google Scholar]