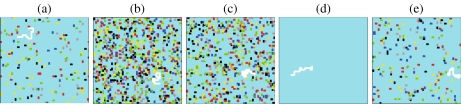
Fig. 6.
Typical configurations of folding–unfolding of a coarse-grained protein suspended in water at different temperatures T and high pressures P. The protein is represented as a fully hydrophobic chain (in white), surrounded by water molecules (turquoise background). We use different color sticks for HBs in different bonding states. a At high P and high T, the protein unfolds and the number of HBs of the surrounding water is small. b At the same pressure but lower T, the protein collapses into a molten globule state. c At lower T the protein folds, while the surrounding water has a large number of HBs. d At much lower T we observe cold denaturation of the protein when the number of HBs is largely reduced (zero in the configuration represented here). e At higher P the denaturation occurs at higher T, and the mechanism of unfolding seems to be dominated by the reduction of HBs under these conditions