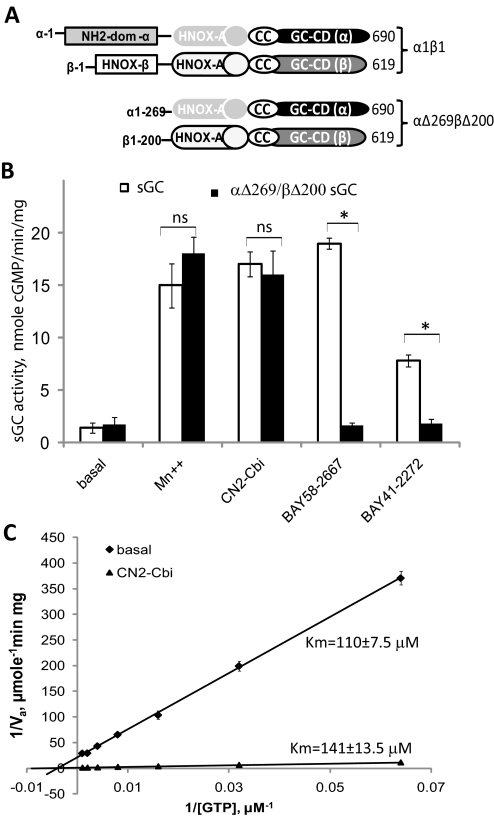
Fig. 4.
CN2-Cbi does not require the sGC regulatory region and does not decrease GTP Km. A, schematic representation of the domain structure of the wild-type α1β1 and the truncated αΔ269βΔ200 sGC. The domain structure is adapted from Ma et al. (2008). Coil-coil elements (CC) and the HNOX-associated PAS-like domain (HNOX-A) are involved in dimerization. The heme-NO/oxygen-binding domain of the β subunit (HNOX-β) binds heme. GC-CD, guanylyl cyclase catalytic domains; NH2-dom-α, amino-terminal domain of the α subunit. B, cGMP-forming activity in 100,000g supernatants of Sf9 cells expressing wild-type (□) or truncated αΔ269/βΔ200 (■) sGC was tested under basal conditions in the presence of 4 mM Mn2+, 100 μM CN2-Cbi, 40 nM BAY58-2667, or 2 μM BAY41-2272. Data are presented as means ± S.D. of two independent preparations assayed in triplicate. ns, difference is not statistically significant; *, p < 0.05. C, effect of CN2-Cbi on the GTP Km. Lineweaver-Burk double-reciprocal plots of the basal (♦) and 100 μM CN2-Cbi (▴)-stimulated sGC activity (Va) are shown. sGC activity was determined using the 7.25 to 2000 μM range of GTP concentrations. Summarized data of two independent experiments performed in triplicate are shown.