Abstract
Natural Killer (NK) lymphocytes, strongly expressing CD56, become abundant in the human uterus three to five days after the mid-menstrual cycle surge in pituitary-derived luteinizing hormone (LH). The primary functions of LH are to initiate final oocyte maturation/ovulation and to contribute to decidualization of the uterine stroma. Decidualization is the transformation of estrogen-primed uterine stromal fibroblasts into large hormone producing cells under the influence of progesterone (P4). Decidual CD56bright (dNK) cells are a distinct, transient, tissue-specific NK cell subset that undergoes proliferation, terminal differentiation, and then death prior to menses. If pregnancy occurs, dNK cells increase during first trimester, then decline and are virtually absent in late pregnancy. In mouse models, pregnancy-associated uterine NK (uNK) cells appear coincident with onset of decidualization during embryonic implantation. Murine uNK cells traffic from the circulation to the anti-mesometrial side of the uterus and migrate to the mesometrial side of each implantation site. Here they proliferate and are implicated in regulation of mid-gestation structural changes to major arteries supplying the placenta, before dying in late gestation. Emerging data indicate that interactions between lymphocytes and endothelial cells within the uterine microenvironment are mediated by classical molecules associated with lymphocyte trafficking in immune surveillance and in response to inflammation. Here, we review factors influencing NK cell trafficking to decidualizing murine and human uteri and the differentiation and functions of these cells within the uterus.
INTRODUCTION
The arrival of a population of NK cells in the mammalian uterus in association with decidualization and pregnancy represents an immunological enigma75. Despite great strides that have been made in defining interactions between maternal and fetal cells in primates and in rodents19;40, the molecules regulating the trafficking of these cells to the uterus remain unclear. The original hypotheses postulated by Sir Peter Medawar to account for success of viviparity included anatomic separation of the mother and fetus, antigenic immaturity of the fetus and/or inertness of the maternal immune system51. It is well established that trophoblast, the cell lineage providing the fetal component of the placenta, separates the fetal and maternal circulations and permits only limited bi-directional cell traffic48;49. In humans, trophoblast expresses a unique and restricted pattern of HLA molecules37;47 which is recognized by inhibitory and activating molecules of the NK receptor gene families known as killer cell immunoglobulin-like receptors (KIR for human)33 or LY49 (mice)60 expressed on CD56+ cells, that are transiently, the predominant uterine lymphocytes of early pregnancy47. Cells of the NK lineage constitute about 15% of peripheral blood lymphocytes but 70% of total lymphocytes in the decidualizing uterus. Although many of these decidual (d)NK cells proliferate in situ, there is mounting evidence that dNK precursors traffic from blood to the uterus in response to hormone-derived signals. In mice, adoptive cell transplantation has demonstrated that the uterine (u)NK cell lineage can be established from peripheral progenitor cells11. Here, we review the recent literature about the adhesion molecules, cytokines, chemokines and hormones thought to be involved in the trafficking of NK cells to the murine uterus.
Lymphocyte Trafficking in Immune Surveillance and Inflammation
Mechanisms regulating lymphocyte homing, such as trafficking of naive T and B cells to secondary lymphoid tissues (lymph nodes (LN) and Peyer’s Patches (PP)), are well described as a four step sequence of events, summarized in Figure 15;7;74;82. First, a sequence of transient adhesive interactions occurs under shear force between adhesion molecules constitutively expressed on circulating lymphocytes (L-selectin) and their ligands [CD34, GlyCAM-1, podocalyxin, Sgp200, collectively known as peripheral node addressin (PNAd) and recognized by the mAb MECA-79] on specialized high endothelial venules (HEV)67. This causes lymphocytes to progressively slow, and roll, bringing the lymphocytes into close contact with endothelium. There, the second step, rapid activation of integrins [αLβ2 (LFA-1) or α4β7 (L-PAM)] occurs in response to tissue-derived chemokines, enabling the third step, binding to endothelial ligands such as ICAM-1 or ICAM-2 in LN or mucosal addressin cell adhesion molecule (MAdCAM) in PP. Finally, cells undergo transendothelial migration along a chemokine gradient into tissue. Tightly regulated chemokines signals permit trafficking of specific subsets of lymphocytes into microdomains of lymphoid tissues, such that B cells migrate at follicular sites of LN and PP while T cells egress at interfollicular areas6;14;30;59;83.
Figure 1.
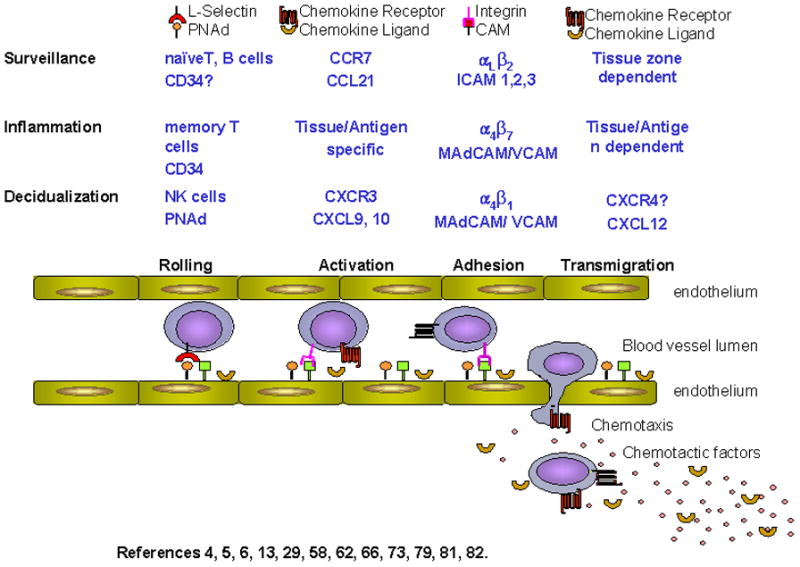
Lymphocyte Extravasation into Tissues
This classic homing pathway, while focusing on naive T and B cell recruitment, also applies to other leukocytes; specifically, migration of memory lymphocytes, dendritic cells, neutrophils and other leukocytes to tertiary sites in response to specific inflammation-induced chemokine signals63. Inflammatory cytokines, secreted in response to injury or pathogens, simultaneously up-regulate expression of adhesion molecules such as E-selectin, VCAM-1 and ICAM-1 on vascular endothelium in the immediate area to facilitate egress of immune cells to sites of inflammation6. Studies on the expression, regulation and function of adhesion molecules and chemokines in homing of immune cells to the decidualizing uterus during normal pregnancy are limited.
Human Natural Killer Cells
The properties of NK cells found in human endometrium indicate that dNK cells are not simply in-transit, circulating cells. Circulating NK cells are subdivided by expression of CD3, CD16 and CD56. The majority of circulating NK cells express CD16 strongly and CD56 weakly (CD56dim), but about 10% of blood NK cells have low expression of CD16 and strongly express CD56 (CD56bright)66. CD3 expressing CD56+ cells are designated NK-T cells as they also express the αβ T cell receptor, thus considered T cells rather than NK cells. The CD56bright subset is further differentiated from the dim subset by expression of high affinity IL-2R, higher expression of L-selectin and α4β1 integrins, but lower expression of αLβ2 27. Table 1 summarizes expression of molecules on NK cell subsets.
Table 1.
Expression of surface molecules on NK cell subsets
| Molecule | Blood | UNK | |
|---|---|---|---|
| CD56Dim | CD56Bright | CD56Bright | |
| CD2 | ✓ | ✓ | ✓ |
| CD11a (LFA-1) | ✓ ✓ | ✓ | ✓ |
| CD16 | ✓ | × | × |
| CD18 (β2) | ✓ | ✓ | ✓ |
| CD29 (β1) | ✓ | ✓ | ✓ ✓ |
| CD49a (α1) | ✓ | ✓ | ✓ ✓ |
| CD49d (α4) | ✓ | ✓ | ✓ ✓ |
| CD57 | ✓ | ✓ ✓ | × |
| CD62L | ✓ | ✓ ✓ ✓ | × |
| CD69 | × | × | ✓ |
| CD94 | ✓ | ✓ ✓ | ✓ ✓ |
| KIR | ✓ | × | ✓ ✓ |
| IL 2Rβ | ✓ | ✓ ✓ | ✓ ✓ |
| c-kit | × | ✓ | ✓ |
CD56 bright and dim cells also differ in their biologic functions; CD56dim cells have greater lytic ability, while CD56bright cells have immunoregulatory roles mediated through secretion of cytokines17. NK cell function is regulated by receptors that recognize MHC class I molecules on target cells. Two structurally distinct families of NK receptors have been identified, KIRs, which are inhibitory receptors and the C-type lectin-like family (CD94/NKG2A, NKG2D, CD69) which act as activating receptors. Human dNK cells are distinguished from peripheral blood NK cells by expression of CD56 at ten-fold higher levels, lack of expression of CD16 and reduced lytic ability53, despite expression of a similar repertoire of activation markers23;33. In contrast to circulating CD56bright cells that express high levels of the L-selectin homing receptor26, dNK cells lack L-selectin69;72;73, perhaps due to shedding during transit through endothelium80.
Despite apparent hormonal regulation of dNK cell appearance in the uterus, hormone effects are thought to be indirect, since they do not express receptors to progesterone (PR)39 or the predominant estrogen receptor (ERα), but do express ERβ32. Indeed, dNK cells do not respond to hormonal stimulation in culture by proliferation, cytokine secretion or gain in lytic ability43. Similarly, evidence of expression of ER or PR isoforms or luteinizing hormone receptor on blood NK cells is inconsistent.
In mammals with hemochorial placentation, which includes both primates and rodents, the maintenance of NK cells in decidualizing uterus is thought to require IL-1541. CD56+ cells have constitutive expression of the α β and γ chains of the IL15R71 to drive further differentiation and proliferation44;81. IL-15 is synthesized by endometrial stromal cells in the decidua and its production appears to be regulated by P458. Transcripts of IL-15 in human perivascular endometrial stromal cells is detected primarily during the secretory phase of the menstrual cycle44 and immediately prior to and after the onset of menses, and during the late proliferative phase13. In pregnancy, IL-15 mRNA is detected in endometrial stromal cells and in endometrial endothelium during the first trimester44. Interestingly, incubation of CD16− NK cells from peripheral blood with IL-15, results in increased expression of CD5615 and development of a chemokine receptor repertoire similar to that found on dNK cells31.
Potential Mechanisms for Trafficking of NK Cells to the Decidualizing Uterus
Despite the reported paucity of NK cell migration to secondary lymphoid tissues8, circulating CD56bright cells express the necessary adhesion molecules and chemokine receptors to support homing to across HEV in secondary lymphoid organs (Figure 2, Table 1). CD56bright cells have been found at low frequency in peripheral LN where they are speculated to provide a physiological link between adaptive and innate immune response s through secretion of IL-226. Like other immune and inflammatory cells, NK cells leave the circulation to enter tissues through interactions with the vessel wall under flow conditions to arrest and transmigrate. NK cells respond to a variety of cytokines and chemokines, including IL-12, IFNα and β, CCL2, 3, 4, 5, 7, 8, CXCL8, and CX3CL19;42. Thus, it is plausible that L-selectin, α4β1, α4β7, and αLβ2 integrins expressed by NK cells function during trafficking of NK cells or their precursors to the decidualizing uterus. We have taken advantage of the ability of adhesion molecules to recognize ligands across species to investigate trafficking potential of both human and murine NK cells using mouse tissue-based cell adhesion assays.
Figure 2.
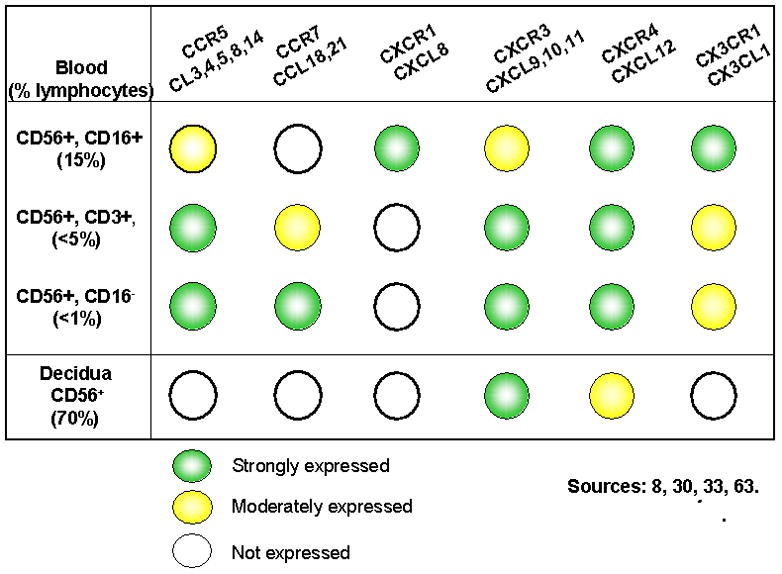
Chemokine Receptors on CD56+ cell subsets
MURINE INVESTIGATIONS
Mice have a short 5 day estrous cycle and no post-ovulatory decidualization. Uterine decidualization is associated with blastocyst implantation at gestation day 454;68. Implantation and primary uterine decidualization occur on the anti-mesometrial side of the uterus, the side opposite the major vascular connections to the uterine artery54;68. Decidualization rapidly moves towards the mesometrial side of the uterus and at gestation days 5–7 involves the entire implantation site. With subsequent growth of the fetus, most of the anti-mesometrial decidua is lost and the mesometrial decidua that remains associated with the developing placenta is given the term decidua basalis. An influx of NK cells, considered to be immature because they lack cytoplasmic granules, is observed within 24 hr of implantation61. These cells are first detected in the secondary, mesometrial decidua. Unlike their human counterparts, murine NK cell subsets have been discriminated by morphology and carbohydrate biochemistry rather than by immune surface phenotype61. By gestation day 6, large numbers of uNK cells are present, they are rapidly proliferating and acquiring their mature appearance of very large, heavily granulated lymphocytes. By gestation day 8, a few uNK cells are dying and by gestation day 12, cell division has ceased and most uNK cells are degenerating20;61.
Functions of uNK Cells
Histological comparisons of implantation sites in alymphoid (NK−, T−, B−) mice to those in T and B cell deficient mice and to those in normal mice suggested that the NK cell influx contributed to maintenance of a fully differentiated decidua. Perhaps more importantly, uNK cells appeared to be essential for induction of the normal, pregnancy-associated structural changes in the convoluted arteries supplying placentae, that are called spiral arteries29. This conclusion was confirmed by adoptive cell transfer from T and B cell deficient SCID mice to alymphoid mice which established the uNK cell lineage and function. Thus, in mice, NK cells are essential for normal vascular development of implantation sites28. Furthermore, the effects of uNK cells on the vasculature appeared to be mediated through uNK cell production of IFNγ since structural changes to vessels observed when pregnant alymphoid mice were reconstituted with NK cells could be fully replicated in alymphoid mouse pregnancies by cell free injections of mrIFN 2.
It is not clear whether uNK cells activate their terminal differentiation and cytokine production programs prior to, coincident with, or following their arrival in the decidualizing uterus. By gestation day 6 pregnancy-induced gains in expression of LY49 inhibitory receptors on uNK cells is complete 60. However, transcription factors Eomes and t-bet which regulate expression of IFNγ, continue to rise as uNK cells differentiate from small, agranular cells (gd6) to mature, heavily granulated cells 78. Uterine NK cell-derived IFNγ can be demonstrated by gestation day 6 and accounts for ~90% of the IFNγ produced in decidua basalis1. In addition to providing the IL-15 survival signal, decidualized mouse uterus also provides redundant pathways of regulatory signaling (ie, IL-12, 18, 23 and 27) for induction of IFNγ85, suggesting that entry to the uterus triggers uNK cell function.
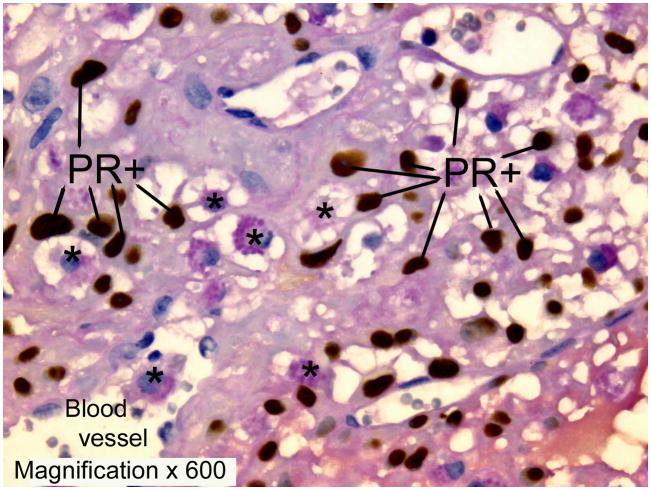
Since the effects of uNK cells are mediated through IFNγ regulated genes in target cells, the midgestation distribution of murine uNK cells suggests that there are multiple target cell types with differing regulated genes. About 10% of midgestation uNK cells appear in the lumens of the spiral arteries, suggesting endothelium is a target. Another 25% are embedded within the vascular smooth muscle layers of the decidual arteries, suggesting smooth muscle cells are major targets. Most of the remaining cells are associated with decidual stroma and matrix, suggesting fibroblast cells are also targets. Many uNK cells in the decidua basalis are associated with larger, irregularly shaped cells that express PR (Figure 3)55. The identity of these PR+ cells is not yet known but the positional relationships are reminiscent of those with DC-SIGN+ dendritic cells in human endometrium and suggest regulatory interactions35. Murine uNK cells are also found at low frequency within the placenta, where fetally-derived trophoblast cells are potential targets for IFNγ regulated changes in gene transcription62;76.
Figure 3.
Sources of uNK Cell Progenitors
Murine uterine horn segment transplantation was used to determine that normal uterus (ie the graft) did not contain resident uNK precursor cells11. A uterine horn segment from a normal mouse was anastomosed into the horn of a mouse genetically deficient in NK and uNK cells, prior to breeding of the host mouse. During the resultant pregnancy, the graft decidualized but developed no uNK cells. Uterine NK containing grafts were found when the recipients were normal mice, indicating that precursors of uNK cells traffic from the circulation to the uterus at decidualization. Sources of uNK cell progenitors was further investigated in vivo, using adoptive transfer of cells from bone marrow, thymus, neonatal liver, spleen, and peripheral and mesenteric LN from virgin and pregnant wildtype (B6) mice into NK cell deficient mice11. All tissues, with the exception of LN that drain the pelvic organs (collected from pregnant donors), were able to re-constitute the uNK cell lineage. Splenic cells derived from pregnant donors were the most competent source of uNK precursors. These results suggested a role for pregnancy or the hormones of pregnancy in regulating uNK cell precursor trafficking from reservoirs such as the spleen into the circulation and their retention in the uterus.
Adhesion Molecule Involvement in uNK Cell Recruitment to Decidua Basalis During Pregnancy
Since immune cells during pregnancy display many immunological markers associated with immune surveillance and inflammation (increased CD11b, CD14, and CD69 as well as increased intracellular reactive oxygen species), we postulated that molecules known to orchestrate leukocyte trafficking to inflamed sites also contribute to leukocyte homing to the uterus during early pregnancy24;25;70. The murine decidua basalis hosts an elegantly orchestrated succession of maternal leukocyte and fetal trophoblastic homing events that appear to be directed by vascular addressins on decidual endothelium. NK cells are found mainly in the central decidua basalis where the vascular endothelium expresses VCAM-1, a ligand for the α4β1 integrins present on uNK cells. However, MAdCAM-1 (a ligand for α4β7 and α4β1 integrins) PNAd, E-selectin and P-selectin were not found in the central decidua basalis using immunohistochemical techniques45. Endothelium at the leading edge of invading trophoblast expresses E-selectin, and attracts neutrophils from the circulation. The vascular zone, which separates the decidua basalis from the anti-mesometrial decidua expresses MAdCAM-1 and P-selectin and hosts monocytes. Thus, subcompartments within implantation sites in pregnant mice are differentially regulated to express highly specific patterns of adhesion molecules that would selectively recruit different leukocyte subsets from the circulation.
Hormone Effects on Endothelium
To investigate the relative roles of adhesion molecules in mediating trafficking to decidualizing uterus, we assessed lymphocyte adhesion to frozen uterine tissues collected from virgin, pregnant or ovariectomized, hormonally treated B6 mice, in in vitro assays11;12. Gestation or treatment of ovariectomized mice with estradiol (E2), or P4, or E2+P4 promoted gains in adhesive function by endothelial cells in an organ-restricted manner. These gains of function occurred in the central decidua basalis of the uterus and in specialized HEV of secondary lymphoid tissues (LN and PP) but not in squamous epithelium of extralymphoid tissues (pancreas)11. Interestingly, these gains were not seen if lymphocytes were pre-treated with function-blocking antibodies to L-selectin or α4 integrin12, the ligands of which (PNAd and MAdCAM, respectively) are reported to be absent from the decidua basalis45. We speculate either that vascular addressins are functionally displayed on decidual vessels at levels below detection by immunohistochemical techniques or that ligands activated or modified by pregnancy hormones or their products are not recognized by monoclonal antibodies.
Human lymphocytes or murine cell lines with known adhesion molecules were used as indicator cells for these assays. When human NK cells were pre-labelled with a fluorescent antibody to CD56, we found CD56bright cells adhered only to the decidua basalis and were preferentially enriched amongst adherent cells (70% of adherent cells were CD56bright although the starting suspensions contained less than 3% CD56bright lymphocytes). This NK subset is characterized by extraordinarily high levels of L-selectin, as compared to naïve T and B lymphocytes, neutrophils, monocytes and CD56dim lymphocytes26. Both pregnancy and the pregnancy–related hormones enhanced lymphocyte-endothelial interactions. Function-blocking antibodies to PNAd/L-selectin or MAdCAM-1/VCAM/α4 established that observed pregnancy-related enhancement in NK cell adhesion were mediated through these molecules, but the extracellular matrix protein, fibronectin, (which contains integrin binding sites) did not contribute to CD56+ cell adhesion11;12. Although pregnancy increased the total number of CD56bright cells binding to HEV of lymphoid organs, no enrichment of this subset was observed relative to the total leukocyte population. These findings, together with evidence that identical molecular interactions between lymphocytes and endothelium are regulated by both pregnancy or pregnancy-associated hormones strongly suggests that decidual endothelium is a unique, extralymphoid site specialized for recruitment of NK cells. Our histological observations that uNK cells appear to be in transit across endothelium in arteries and are localized in large numbers around arteries, but not veins in rodent implantation sites raises the possibility that unique sites of extravasation are involved in uterine trafficking (Croy, unpublished). Intravital microscopy is required to establish the direction of transit through these vessels.
Hormone Effects on Lymphocytes
In concert with endothelial changes in response to pregnancy and pregnancy hormones, a coordinated change in adhesive capability of lymphocytes from treated mice was observed. Adhesion by splenocytes from pregnant and hormone-treated ovariectomized mice was significantly higher than by splenocytes from virgin or ovariectomized and-placebo treated mice on a common LN substrate. The enhanced adhesion was blocked by pre-treatment of the splenocytes with function blocking antibodies to L-selectin12. A role for L-selectin is further supported by recent studies showing that lymphocytes from L-selectin-deficient mice exhibit impaired short-term trafficking (0.5–1 h) to lymphoid and uterine tissues in pregnant animals84. It is tempting to speculate that pregnancy or their associated hormones activate receptors on lymphocytes such that they increase in trafficking potential to uterus and secondary lymphoid organs.
Role of Chemokines and their Receptors in NK Cell Homing to the Uterus
Individualized patterns of chemokine receptor expression have been described on NK cell subsets from blood9;34;46 as well as from decidua 31;46;64 and these are summarized in Figure 2. Histological and morphometric analyses of implantation sites in mice genetically deleted for the chemokine receptors CCR2 and CCR5 (ligands MCP-2, MIP-1β and RANTES) showed no alteration in uNK cell numbers, positioning or function (assessed as spiral artery modification) when these chemokine mediated pathways were eliminated10. The chemokine receptor CXCR3 contributes to leukocyte chemotaxis and is expressed on a wide variety of lymphocytes including human CD16−CD56bright NK cells and dNK cells31 and mouse uNK cells84. Compared with CD16+CD56dim NK cells, CD16−CD56bright NK cells express higher levels of CXCR38 and respond more vigorously to its ligands, Mig (CXCL9) and IP-10 (CXCL10) which are present in decidua31. Since these chemokines are IFNγ induced, it seems probable that uNK cells promote their own enrichment in the decidua, through secretion of IFNγ. In mice, genetic loss of CXCR3 function resulted in fewer NK cells only in the mural aspect of the decidua basalis, a microdomain normally enriched in more immature, proliferating uNK cells84. This was associated with some but not total impairment of spiral artery modification. These studies, summarized in Table 2, suggest that L-selectin-based interactions may initiate homing of leukocytes while CXCR3-based interactions participate in positioning of uNK cells within the uterine microdomains during early pregnancy.
Table 2.
Effect of gene deletion on murine uNK cell homing, distribution and function at gestation day 10.
| Deleted Gene Products | uNK Cell Density | uNK Cell Positioning | Spiral Artery Modification | Reference |
|---|---|---|---|---|
| Trafficking Molecules | ||||
| L-selectin | Reduced <6h | Normal | Normal | 84 |
| CCR2 | Normal | Normal | Normal | 10 |
| CCR5 | Normal | Normal | Normal | 10 |
| CXCR3 | Reduced | Deficit in MLAp | Slightly impaired | 84 |
| Activation Molecules | ||||
| IL-12 p40 (B6) | Normal | Normal | Impaired | 18 |
| IL18 (B6) | Normal | Normal | Impaired | 85 |
| IL-12−/−/18−/− (B6) | Normal | Normal | Impaired | |
| IL-15 (B6) | No uNK cells | No uNK cells | None | 1 |
PREGNANCY HORMONES, DECIDUALIZATION AND CHEMOTAXIS
Effects of Progesterone On Stromal Cells, Endothelial Cells and Lymphocytes
Uterine decidualization is dependent upon P4 activation of E2 primed endometrial stroma50. Progesterone also regulates decidual production of cytokines such as IL-1558;81, leukemia inhibitory factor (LIF), required for implantation and IL-11 which contributes to further decidualization in an autocrine manner21;65. Progesterone induces stromal cell production of CXCR3 ligands, CXCL10 and CXCL11 at the time NK cells are known to accumulate at implantation sites (Figure 3)42. Chemokine receptors which are greatly up-regulated in decidua during the luteal (P4 dominant) phase include CXCR1, CCR2b and CCR522. Protein products such as renin, prolactin and insulin-like growth factor binding protein-1 (IGFBP-1) and extra-cellular matrix proteins, laminin and fibronectin, established as a peri-cellular rim, are also produced by decidua under the influence of P4 to facilitate implantation and invasion of trophoblast36. Thus major roles for P4 include induction of decidualization, induction of chemokines capable of recruiting NK cells and induction of IL-15 to drive and maintain terminal uNK cell differentiation.
Effects of Estradiol on Endothelial Cells and Lymphocytes
The ovarian hormone estrogen exerts effects through interactions with ER found in many cell types, including endothelial cells throughout the cardiovascular system. Estradiol induces vasodilation or relaxation in vessels52;77. These effects are particularly profound in the pregnant uterus, where uterine arteries elongate and become tortuous while undergoing major dilation with the result of having increased flow of maternal nutrients to the feto-placental unit. A direct role of E2 in NK cell activation and/or differentiation is controversial. Bone marrow transplant experiments in which alymphoid mice received grafts from mice deficient in ERα or ERβ showed apparently normal differentiation of the uNK cell lineage and its full function as assessed by spiral artery modification3, indicating that E2 was not essential to recruitment or differentiation of uNK cells.
Model of dNK Precursor Cell Recruitment to the Human Uterus
From information provided by murine models, human uterine immunohistology and in situ hybridizations and assays of human blood and uterine NK cell functions, a model of redundancy in cell origin and in recruitment pathways has emerged for uNK cells. To date, the only non redundant processes defined are the absolute requirement for IL-15 in early uNK cell differentiation, the absolute requirement for co-differentiation of decidua and the need for uNK cell derived IFNγ to effect spiral artery modification. Figure 4 demonstrates our proposed model of uNK cell trafficking; under the influence of hormones or hormone-derived factors uNK cell precursors enter the circulation from various lymphoid reservoirs, particularly spleen during pregnancy. Once circulating, CD56bright cells enter LN where they interact with other immune cells, priming them to respond to chemotactic signals from the decidualizing uterus/conceptus. Egress from blood vessels is accomplished using classic adhesion molecules, which includes but is not restricted to L-selectin and α4 integrin, to adhere to endothelium in the decidua. It appears probable that at implantation, this initially occurs anti-mesometrially at the site of implantation and first decidualization, but that as decidualization rapidly progresses, entry is restricted to the decidua basalis by gestation day 6. Final uNK cell positioning in the decidua and mural regions is effected by CXCR4 and CXCR3 molecules respectively on NK cells.
Figure 4.
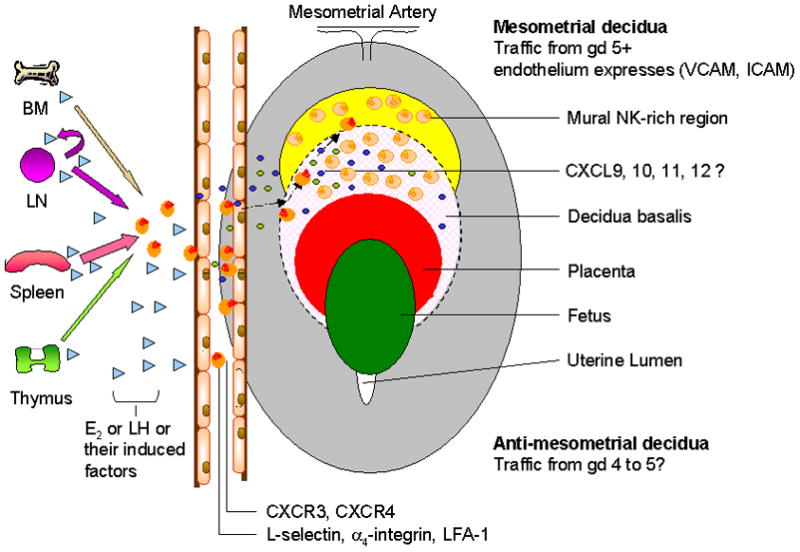
We conclude that trafficking of pro-uNK cells to the uterus utilizes well-established trafficking molecules in response to a unique set of stimulating molecules consisting of hormones (or their induced factors) and specific combinations of chemokines to correctly attract and position uNK cells within the uterus. Aberrant regulation of these pathways may contribute to implantation failure or arrest and subsequent failure of developing conceptuses.
Acknowledgments
We thank J.E. Lewis, A. Simpson, K. Hatta, S. Burke and especially W.C. Wang for technical assistance. We appreciate the assistance of C. Peralta in preparation of the figures.
Supported by NSERC, CIHR, OMAFRA, Ontario Women’s Health Scholar Award and NIH.
Abbreviations
- B6
Wildtype C57BL6 mice
- dNK
decidual Natural Killer cells of human pregnancy
- E2
estradiol
- ER
estrogen receptor
- HEV
high endothelial venule
- ICAM-1
intercellular adhesion molecule-1
- LFA-1
leukocyte function associated molecule -1
- LIF
leukemia inhibitory factor
- LH
luteinizing hormone
- LN
Lymph Node
- mAb
monoclonal antibody
- MAdCAM
mucosal addressin cell adhesion molecule
- NK
Natural Killer cell
- PNAd
peripheral lymph node addressin
- PP
Peyers Patches
- P4
progesterone
- PR
progesterone receptor
- uNK
uterine NK cell of mouse pregnancy
- VCAM-1
vascular cell adhesion molecule-1
References
- 1.Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod. 1999;61:493–502. doi: 10.1095/biolreprod61.2.493. [DOI] [PubMed] [Google Scholar]
- 2.Ashkar AA, di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borzychowski AM, Chantakru S, Paffaro VA, Yamada AT, He H, Korach KS, Croy BA. Functional analysis of murine uterine natural killer cells genetically devoid of oestrogen receptors. Placenta. 2003;24:403–411. doi: 10.1053/plac.2002.0924. [DOI] [PubMed] [Google Scholar]
- 4.Burrows TD, King A, Loke YW. Expression of adhesion molecules by human decidual large granular lymphocytes. Cell Immunol. 1993;147:81–94. doi: 10.1006/cimm.1993.1050. [DOI] [PubMed] [Google Scholar]
- 5.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 6.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DJ, Debes GF, Johnston B, Wilson E, Butcher EC. Targeting T cell responses by selective chemokine receptor expression. Semin Immunol. 2003;15:277–286. doi: 10.1016/j.smim.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Campbell JJ, Bowman EP, Murphy K, Youngman KR, Siani MA, Thompson DA, Wu L, Zlotnik A, Butcher EC. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7. J Cell Biol. 1998;141:1053–1059. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 10.Chantakru S, Kuziel WA, Maeda N, Croy BA. A study on the density and distribution of uterine Natural Killer cells at mid pregnancy in mice genetically-ablated for CCR2, CCR 5 and the CCR5 receptor ligand, MIP-1 alpha. J Reprod Immunol. 2001;49:33–47. doi: 10.1016/s0165-0378(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 11.Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168:22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- 12.Chantakru S, Wang WC, van den Heuvel M, Bashar S, Simpson A, Chen Q, Croy BA, Evans SS. Coordinate Regulation of Lymphocyte-Endothelial Interactions by Pregnancy-Associated Hormones. J Immunol. 2003;171:1132–1145. doi: 10.4049/jimmunol.171.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chegini N, Ma C, Roberts M, Williams RS, Ripps BA. Differential expression of interleukins (IL) IL-13 and IL-15 throughout the menstrual cycle in endometrium of normal fertile women and women with recurrent spontaneous abortion. J Reprod Immunol. 2002;56:93–110. doi: 10.1016/s0165-0378(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 14.Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 15.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 17.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 18.Croy BA, Ashkar AA, Foster RA, DiSanto JP, Magram J, Carson D, Gendler SJ, Grusby MJ, Wagner N, Muller W, Guimond MJ. Histological studies of gene-ablated mice support important functional roles for natural killer cells in the uterus during pregnancy. J Reprod Immunol. 1997;35:111–133. doi: 10.1016/s0165-0378(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 19.Croy BA, Kiso Y. Granulated metrial gland cells: a natural killer cell subset of the pregnant murine uterus. Microsc Res Tech. 1993;25:189–200. doi: 10.1002/jemt.1070250302. [DOI] [PubMed] [Google Scholar]
- 20.Delgado SR, McBey BA, Yamashiro S, Fujita J, Kiso Y, Croy BA. Accounting for the peripartum loss of granulated metrial gland cells, a natural killer cell population, from the pregnant mouse uterus. J Leukoc Biol. 1996;59:262–269. [PubMed] [Google Scholar]
- 21.Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod. 2002;8:636–643. doi: 10.1093/molehr/8.7.636. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez F, Galan A, Martin JJ, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod. 2003;9:189–198. doi: 10.1093/molehr/gag024. [DOI] [PubMed] [Google Scholar]
- 23.Eidukaite A, Siaurys A, Tamosiunas V. Differential expression of KIR/NKAT2 and CD94 molecules on decidual and peripheral blood CD56bright and CD56dim natural killer cell subsets. Fertil Steril. 2004;81(Suppl 1):863–868. doi: 10.1016/j.fertnstert.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Evans SS, Schleider DM, Bowman LA, Francis ML, Kansas GS, Black JD. Dynamic association of L-selectin with the lymphocyte cytoskeletal matrix. J Immunol. 1999;162:3615–3624. [PubMed] [Google Scholar]
- 25.Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97:2727–2733. doi: 10.1182/blood.v97.9.2727. [DOI] [PubMed] [Google Scholar]
- 26.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 27.Frey M, Packianathan NB, Fehniger TA, Ross ME, Wang WC, Stewart CC, Caligiuri MA, Evans SS. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161:400–408. [PubMed] [Google Scholar]
- 28.Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 1997;56:169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- 29.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S, Peled A, Mandelboim O. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16− human natural killer cells. Blood. 2003;102:1569–1577. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- 32.Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab. 2003;88:440–449. doi: 10.1210/jc.2002-021174. [DOI] [PubMed] [Google Scholar]
- 33.Hiby SE, King A, Sharkey AM, Loke YW. Human uterine NK cells have a similar repertoire of killer inhibitory and activatory receptors to those found in blood, as demonstrated by RT-PCR and sequencing. Mol Immunol. 1997;34:419–430. doi: 10.1016/s0161-5890(97)00032-1. [DOI] [PubMed] [Google Scholar]
- 34.Inngjerdingen M, Damaj B, Maghazachi AA. Expression and regulation of chemokine receptors in human natural killer cells. Blood. 2001;97:367–375. doi: 10.1182/blood.v97.2.367. [DOI] [PubMed] [Google Scholar]
- 35.Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, van Kooyk Y, Kampgen E. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol. 2003;162:887–896. doi: 10.1016/S0002-9440(10)63884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King A. Uterine leukocytes and decidualization. Hum Reprod Update. 2000;6:28–36. doi: 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- 37.King A, Allan DS, Bowen M, Powis SJ, Joseph S, Verma S, Hiby SE, McMichael AJ, Loke YW, Braud VM. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–1631. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 38.King A, Balendran N, Wooding P, Carter NP, Loke YW. CD3- leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol. 1991;1:169–190. doi: 10.1155/1991/83493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King A, Gardner L, Loke YW. Evaluation of oestrogen and progesterone receptor expression in uterine mucosal lymphocytes. Hum Reprod. 1996;11:1079–1082. doi: 10.1093/oxfordjournals.humrep.a019300. [DOI] [PubMed] [Google Scholar]
- 40.King A, Loke YW. Uterine large granular lymphocytes: a possible role in embryonic implantation? Am J Obstet Gynecol. 1990;162:308–310. doi: 10.1016/0002-9378(90)90375-h. [DOI] [PubMed] [Google Scholar]
- 41.King A, Wellings V, Gardner L, Loke YW. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol. 1989;24:195–205. doi: 10.1016/0198-8859(89)90060-8. [DOI] [PubMed] [Google Scholar]
- 42.Kitaya K, Nakayama T, Daikoku N, Fushiki S, Honjo H. Spatial and temporal expression of ligands for CXCR3 and CXCR4 in human endometrium. J Clin Endocrinol Metab. 2004;89:2470–2476. doi: 10.1210/jc.2003-031293. [DOI] [PubMed] [Google Scholar]
- 43.Kitaya K, Yasuda J, Nakayama T, Fushiki S, Honjo H. Effect of female sex steroids on human endometrial CD16neg CD56bright natural killer cells. Fertil Steril. 2003;79(Suppl 1):730–734. doi: 10.1016/s0015-0282(02)04818-5. [DOI] [PubMed] [Google Scholar]
- 44.Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H. IL-15 expression at human endometrium and decidua. Biol Reprod. 2003;63:683–687. doi: 10.1095/biolreprod63.3.683. [DOI] [PubMed] [Google Scholar]
- 45.Kruse A, Merchant MJ, Hallmann R, Butcher EC. Evidence of specialized leukocyte-vascular homing interactions at the maternal/fetal interface. Eur J Immunol. 1999;29:1116–1126. doi: 10.1002/(SICI)1521-4141(199904)29:04<1116::AID-IMMU1116>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 46.Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, Greenberg HB, Hodge MR, Wu L, Butcher EC, Campbell JJ. Alterations in the expression of homing-associated molecules at the maternal/fetal interface. Am J Pathol. 2002;160:347–355. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loke YW, King A. Decidual natural-killer-cell interaction with trophoblast: cytolysis or cytokine production? Biochem Soc Trans. 2000;28:196–198. doi: 10.1042/bst0280196. [DOI] [PubMed] [Google Scholar]
- 48.Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, Nelson JL. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41–47. doi: 10.1172/JCI6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marleau AM, Greenwood JD, Wei Q, Singh B, Croy BA. Chimerism of murine fetal bone marrow by maternal cells occurs in late gestation and persists into adulthood. Lab Invest. 2003;83:673–681. doi: 10.1097/01.lab.0000067500.85003.32. [DOI] [PubMed] [Google Scholar]
- 50.Maslar IA. The progestational endometrium. Sem Reprod Immunol. 1988;6:115–128. [Google Scholar]
- 51.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–338. [Google Scholar]
- 52.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002;90:3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 53.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 54.Mossman HW. Structural changes in vertebrate fetal membranes associated with the adoption of viviparity. Obstet Gynecol Annu. 1974;3:7–32. [PubMed] [Google Scholar]
- 55.Mote PA, Johnston JF, Manninen T, Tuohimaa P, Clarke CL. Detection of progesterone receptor forms A and B by immunohistochemical analysis. J Clin Pathol. 2001;54:624–630. doi: 10.1136/jcp.54.8.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183–3191. [PubMed] [Google Scholar]
- 57.Nishikawa K, Saito S, Morii T, Hamada K, Ako H, Narita N, Ichijo M, Kurahayashi M, Sugamura K. Accumulation of CD16-CD56+ natural killer cells with high affinity interleukin 2 receptors in human early pregnancy decidua. Int Immunol. 1991;3:743–750. doi: 10.1093/intimm/3.8.743. [DOI] [PubMed] [Google Scholar]
- 58.Okada H, Nakajima T, Sanezumi M, Ikuta A, Yasuda K, Kanzaki H. Progesterone enhances interleukin-15 production in human endometrial stromal cells in vitro. J Clin Endocrinol Metab. 2000;85:4765–4770. doi: 10.1210/jcem.85.12.7023. [DOI] [PubMed] [Google Scholar]
- 59.Pachynski RK, Wu SW, Gunn MD, Erle DJ. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin alpha 4 beta 7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J Immunol. 1998;161:952–956. [PubMed] [Google Scholar]
- 60.Paffaro VA, Jr, He H, Yamada AT, Croy BA. LY49 gene expression by uterine natural killer cells during mouse pregnancy. 2005 submitted. [Google Scholar]
- 61.Paffaro VA, Jr, Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta. 2003;24:479–488. doi: 10.1053/plac.2002.0919. [DOI] [PubMed] [Google Scholar]
- 62.Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- 63.Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 64.Red-Horse K, Drake PM, Gunn MD, Fisher SJ. Chemokine ligand and receptor expression in the pregnant uterus:reciprocal patterns in complementary cell subsets suggest functional roles. Am J Pathol. 2001;159:2199–2213. doi: 10.1016/S0002-9440(10)63071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robb L, Dimitriadis E, Li R, Salamonsen LA. Leukemia inhibitory factor and interleukin-11: cytokines with key roles in implantation. J Reprod Immunol. 2002;57:129–141. doi: 10.1016/s0165-0378(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 66.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438. [PubMed] [Google Scholar]
- 67.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 68.Rossant J, Tam PP. Emerging asymmetry and embryonic patterning in early mouse development. Dev Cell. 2004;7:155–164. doi: 10.1016/j.devcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 69.Ruck P, Marzusch K, Kaiserling E, Horny HP, Dietl J, Geiselhart A, Handgretinger R, Redman CW. Distribution of cell adhesion molecules in decidua of early human pregnancy. An immunohistochemical study. Lab Invest. 1994;71:94–101. [PubMed] [Google Scholar]
- 70.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 71.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci U S A. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Searle RF, Jones RK, Bulmer JN. Phenotypic analysis and proliferative responses of human endometrial granulated lymphocytes during the menstrual cycle. Biol Reprod. 1999;60:871–878. doi: 10.1095/biolreprod60.4.871. [DOI] [PubMed] [Google Scholar]
- 73.Slukvin II, Chernyshov VP, Merkulova AA, Vodyanik MA, Kalinovsky AK. Differential expression of adhesion and homing molecules by human decidual and peripheral blood lymphocytes in early pregnancy. Cell Immunol. 1994;158:29–45. doi: 10.1006/cimm.1994.1254. [DOI] [PubMed] [Google Scholar]
- 74.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 75.Stewart IJ. Granulated metrial gland cells in ‘minor’ species. J Reprod Immunol. 1998;40:129–146. doi: 10.1016/s0165-0378(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 76.Stewart IJ, Mukhtar DD. A scanning electron microscopy study of interactions between mouse granulated metrial gland cells and placental trophoblast cells in vitro. J Anat. 1994;184(Pt 1):153–156. [PMC free article] [PubMed] [Google Scholar]
- 77.Sullivan TRJ, Karas RH, Aronovitz M, Faller GT, Ziar JP, Smith JJ, O’Donnell TFJ, Mendelsohn ME. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest. 1995;96:2482–2488. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tayade C, Fang Y, Black GP, Paffaro VA, Jr, Croy BA. Transcription of Eomes and T-bet during differentiation and effector function development of mouse uterine natural killer cells. 2005 doi: 10.1189/jlb.0305142. submitted. [DOI] [PubMed] [Google Scholar]
- 79.Umekage H, Saito S, Morikawa H. Enhancement by stem cell factor of interleukin-2 (IL-2)-induced DNA synthesis in human decidual. J Reprod Immunol. 1998;40:1–24. doi: 10.1016/s0165-0378(98)00028-x. [DOI] [PubMed] [Google Scholar]
- 80.Venturi GM, Tu L, Kadono T, Khan AI, Fujimoto Y, Oshel P, Bock CB, Miller AS, Albrecht RM, Kubes P, Steeber DA, Tedder TF. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity. 2003;19:713–724. doi: 10.1016/s1074-7613(03)00295-4. [DOI] [PubMed] [Google Scholar]
- 81.Verma S, Hiby SE, Loke YW, King A. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod. 2000;62:959–968. doi: 10.1095/biolreprod62.4.959. [DOI] [PubMed] [Google Scholar]
- 82.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 83.Warnock RA, Campbell JJ, Dorf ME, Matsuzawa A, McEvoy LM, Butcher EC. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer’s patch high endothelial venules. J Exp Med. 2000;191:77–88. doi: 10.1084/jem.191.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie X, Kang J, Anderson LN, He H, Lu X, Croy BA. Analysis of the Contributions of L-Selectin and CXCR3 in Mediating Leukocyte Homing to Pregnant Mouse Uterus. Am J Reprod Immunol. 2005;53:1–12. doi: 10.1111/j.1600-0897.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 85.Zhang JH, He H, Borzychowski AM, Takeda K, Akira S, Croy BA. Analysis of cytokine regulators inducing interferon production by mouse uterine natural killer cells. Biol Reprod. 2003 Aug;69(2):404–11. doi: 10.1095/biolreprod.103.015529. Epub. 2003. Mar 2004, 69, 404–411. [DOI] [PubMed] [Google Scholar]