Abstract
Background
It is well established that concomitant aortic valve replacement (AVR) and coronary artery bypass grafting (CABG) has a higher operative mortality rate than isolated AVR. However, studies report conflicting results on the long-term mortality. The aim of this prospective study was to explore and compare the outcomes and risk factors of isolated AVR and concomitant AVR and CABG in a consecutive Dutch patient population.
Methods
From January 2001 through January 2010, 332 consecutive patients underwent AVR with or without CABG at a single institution (197 isolated AVR and 135 concomitant AVR and CABG). A multivariate Cox proportional hazard analysis was performed to determine the independent risk factors for long-term mortality after aortic valve replacement.
Results
All 332 consecutive, referred patients who underwent aortic valve surgery were followed for up to 10 years. Median follow-up length was 48 months. The population had a median age of 73 years (IQR 65–78) and predominantly consisted of males (62%). Patients in the combined AVR and CABG group were older, had worse cardiac risk profiles and had worse preoperative cardiac statuses than those receiving isolated AVR. Five-year survival was 85% in AVR and 73% in AVR-CABG (p-value 0.012). Independent risk factors for mortality were higher creatinine values, previous CABG and increasing age.
Conclusion
Unselected, consecutive patients who underwent aortic valve replacement surgery and who received concomitant bypass surgery between 2001–2010 had higher 5-year mortality than their counterparts without CABG. Prior CABG, renal function, age but not concomitant CABG remained independently associated with increased mortality. Finally, the observed mortality rate in this consecutive patient group compared favourably with preoperative risk assessment using the EuroSCORE.
Keywords: Aortic valve replacement, Coronary artery bypass grafting, Long term, Outcome, Survival, EuroSCORE, Concomitant, Valve, Surgery, AVR, CABG
Introduction
Aortic valve replacement (AVR) is a procedure with a serious risk of adverse outcomes. In order to make a well-informed decision whether to opt for surgery or for other available treatment, the prediction of these outcomes is essential. The operative risk can be estimated with several well-validated risk models such as the European System for Cardiac Operative Risk Evaluation (EuroSCORE), the risk calculator of the Society of Thoracic Surgeons (STS) and the Ambler risk score [1–5]. The Ambler and STS risk models show that concomitant coronary artery bypass grafting (CABG) has a higher operative mortality than isolated AVR. The EuroSCORE, however, does not distinguish between isolated AVR and AVR with concomitant CABG. Rather, it provides an estimate of thoracic surgery in general.
Despite this well-validated evidence on the operative mortality, reports comparing the long-term outcome for isolated AVR and concomitant AVR and CABG are scarce. The little evidence that does exist is conflicting. Jones et al. and Kvidal et al. both reported patients receiving concomitant CABG to have a higher mortality rate in the long term than patients receiving isolated AVR [6, 7]. Melby et al. examined elderly patients and found concomitant CABG to actually improve long-term survival [8]. He et al. studied older patients as well, but did not find concomitant CABG to independently predict mortality [9]. To contribute towards reaching consensus on this matter, we retrospectively examined the risk factors for mortality in the long term, with specific interest in concomitant CABG.
Results on the long-term outcomes of unselected and consecutive series of patients in need of AVR or AVR-CABG are especially sparse in the Netherlands. Therefore, a second goal was to provide Dutch clinicians with these unselected outcome data and compare these data with other international studies. With this in mind, we examined a general population and did not restrict our study to specific subgroups such as octogenarians.
Methods
Population
Between January 2001 and January 2010, 332 consecutive patients were referred from the Medical Center Alkmaar (Alkmaar, the Netherlands) to the VU University Medical Center (Amsterdam, the Netherlands) where they received AVR. Of these, 197 patients received isolated AVR (the AVR group) and 135 patients underwent concomitant AVR and CABG (AVR-CABG group). Indication for AVR was severe and symptomatic aortic valve disease with either aortic valve stenosis, regurgitation or a mixture of both. Preoperative coronary angiography was performed in all cases to determine the presence of coronary artery disease. If significant coronary disease was present, patients received concomitant CABG. To conclude the surgical indication, patients were discussed in a multidisciplinary heart team meeting. Patients who underwent procedures other than isolated AVR or concomitant AVR and CABG were not included in this study.
Patient and operative characteristics were prospectively collected for quality control purposes. The logistic EuroSCORE was calculated using the online calculator (http://www.EuroSCORE.org/calc.html) [5]. In March and April 2011, the database was completed with mortality status and cause of death by reviewing hospital records. If mortality status or cause of death was missing, general practitioners were contacted in order to complete the data. Cause of death was categorised into cardiovascular death and non-cardiovascular death. All deaths were considered cardiovascular unless an unequivocal non-cardiac cause of death could be established. As such, both unwitnessed and sudden death were classified as cardiovascular death. If a non-cardiovascular diagnosis suggested death was imminent, death was classified as non-cardiovascular. Furthermore, the aetiology of the cause of death was recorded for all deaths. We considered the cause of death for residents of a nursing home to be death due to frailty.
Surgical procedures
All surgical procedures were performed by cardiothoracic surgeons of the VU University Medical Center. All procedures were performed using cardiopulmonary bypass and mild systemic hypothermia (30 to 34°C). Myocardial protection was achieved with cold blood cardioplegia. The procedures were performed using either a standard or partial median sternotomy. The operating surgeon selected the type of valve prosthesis. Both bioprostheses and bileaflet mechanical heart valves were used. CABG was performed using the left internal mammary artery (LIMA), the right internal mammary artery (RIMA) and/or venous grafting.
Statistical analysis
All continuous data were expressed as mean ± 1 standard deviation. For continuous data, the median with the interquartile range (IQR) were also displayed. Nominal and ordinal variables were expressed as actual numbers and percentages of the total.
For nominal variables, the Chi-square test or Fisher’s exact test were used to analyse relations between the AVR group and the AVR-CABG group. Continuous variables were analysed using Student’s t-test for normal distributions and Mann-Whitney’s test for non-normal distributions. To test for normal distribution, Kolmogorov-Smirnov’s test was used. Ordinal variables were analysed using Mann-Whitney’s test. To compare classes with respect to AVR and AVR-CABG individually, the z-test with Bonferroni’s correction was used. All p-values <0.05 were considered to be statistically significant.
Kaplan-Meier’s analysis was used to analyse and depict survival over time. The log-rank test was used to compare survival between the AVR group and the AVR-CABG group for 1, 3 and 5 years of follow-up.
Cox proportional hazard analysis (Enter method) was used to examine which of the baseline variables were independent risk factors for all-cause mortality. First, univariate hazard ratios (HR) and their 95% confidence interval (CI) were calculated for all baseline variables. Then variables with a p-value <0.05 were entered into the multivariate analysis. All statistically significant variables in the multivariate analysis were considered independent risk factors.
Database management and statistical analysis were performed using SPSS 14.0 (SPSS Inc, Chicago, IL).
Results
Study population demographics
Demographic and preoperative variables of the two groups (AVR-CABG n = 135 and AVR n = 197) are shown in Table 1. While both groups had more men than women, AVR-CABG had a higher proportion of men than AVR (p-value 0.115). Median age among the total population was 73 years (IQR 65–78). Patients in the AVR-CABG group were older than those in the AVR group (74 vs 69 years; p-value <0.001). They more often had a history of percutaneous transluminary coronary angioplasty (PTCA) and myocardial infarction (MI). The AVR-CABG group had worse cardiac risk profiles, with significantly more hypertension, cardiac family history, hyperlipidaemia and higher creatinine levels. At the time of surgery, they had a worse cardiological status with more angina pectoris, a higher NYHA class and by design of the study more coronary artery disease including left main disease.
Table 1.
Baseline characteristics
| AVR (n = 197 (59%)) | AVR-CABG (n = 135 (41%)) | P-value | |
|---|---|---|---|
| Age (years) | 69 ± 11 | 74 ± 7 | <0.001 |
| Median 70 | Median 75 | ||
| IQR 62–77 | IQR 69–78 | ||
| Sex | |||
| Female | 81 (41%) | 44 (33%) | 0.115 |
| Male | 116 (59%) | 91 (67%) | |
| BMI (kg/m2) | 27 ± 3.9 | 27 ± 4.2 | 0.994 |
| Median 27 | Median 27 | ||
| IQR 24–29 | IQR 24–29 | ||
| COPD | 33 (17%) | 10 (7%) | 0.013 |
| Hypertension | 92 (47%) | 82 (61%) | 0.012 |
| Hyperlipidaemia | 57 (29%) | 67 (50%) | <0.001 |
| Smoking | 29 (15%) | 14 (10%) | 0.246 |
| Angina pectoris | 56 (28%) | 74 (55%) | <0.001 |
| Diabetes mellitus | 23 (12%) | 32 (24%) | 0.004 |
| Intermittent claudication | 8 (4.1%) | 8 (5.9%) | 0.436 |
| Left main stenosis | 1 (0.5%) | 9 (6.7%) | 0.002 |
| Prior CABG | 10 (5.1%) | 8 (5.9%) | 0.737 |
| Prior aorta surgery | 3 (1.5%) | 6 (4.4%) | 0.167 |
| Prior MI | 3 (1.5%) | 20 (14.8%) | <0.001 |
| Prior PCI or PTCA | 7 (3.6%) | 16 (11.9%) | 0.003 |
| Cardiac family history | 38 (19%) | 44 (33%) | 0.006 |
| Prior valve surgery | 3 (1.5%) | 0 (0%) | 0.274 |
| Indication for surgery | 0.372 | ||
| Elective | 34 (17%) | 27 (20%) | † |
| Priority | 98 (50%) | 51 (38%) | * |
| Acute | 65 (33%) | 56 (42%) | † |
| NYHA class | 0.006 | ||
| I | 19 (9.8%) | 5 (3.8%) | * |
| II | 33 (17%) | 10 (7.6%) | * |
| III | 135 (70%) | 114 (87%) | * |
| IV | 7 (3.6%) | 2 (1.5%) | † |
| Preoperative creatinine (μmol/l) | 98 ± 32 | 106 ± 34 | <0.001 |
| Median 92 | Median 102 | ||
| IQR 81–108 | IQR 87–116 | ||
| Prior TIA or CVA | 16 (8.1%) | 14 (10.4%) | 0.483 |
| Left ventricle function | 0.609 | ||
| Good | 144 (74%) | 96 (72%) | † |
| Adequate | 25 (13%) | 15 (11%) | † |
| Moderate | 19 (9.7%) | 15 (11%) | † |
| Poor | 8 (4%) | 8 (6.0%) | † |
* P-value between AVR and AVR + CABG is less than 0.05 for this variable
† P-value between AVR and AVR + CABG is more than 0.05 for this variable
AVR aortic valve replacement, BMI body mass index, CABG coronary artery bypass grafting, COPD chronic obstructive pulmonary disease, CVA cerebrovascular accident, MI myocardial infarction, NYHA New York Heart Association, PCI percutaneous coronary intervention, PTCA percutaneous transluminary coronary angiography, TIA transient ischaemic attack
Operative characteristics are displayed in Table 2. Patients in the AVR-CABG group had a higher logistic EuroSCORE than patients in the AVR group (8.2 vs 6.6%; p-value 0.007). Their cross-clamp time (108 vs 73 min; p-value <0.001) and cardiopulmonary bypass length (153 vs 99 min; p-value <0.001) were longer. They spent more days in the intensive care unit (2.2 vs 1.5 days; p-value <0.001) and had to undergo postoperative rethoracotomy more often (3.6 vs 11.1%; p-value 0.007).
Table 2.
Operative characteristics
| AVR (n = 197 (59.3%)) | AVR-CABG (n = 135 (40.7%)) | P-value | |
|---|---|---|---|
| Additive EuroSCORE | 5.8 ± 2.5 | 6.8 ± 2.4 | 0.001 |
| Median 6.0 | Median 6.0 | ||
| IQR 3.5–7.5 | IQR 5.0–8.0 | ||
| Logistic EuroSCORE | 6.6 ± 7.6 | 8.2 ± 8.3 | 0.007 |
| Median 5.1 | Median 5.8 | ||
| IQR 2.3–8.5 | IQR 4.0–10 | ||
| Cross clamp time (min) | 73 ± 24 | 108 ± 31 | <0.001 |
| Median 76 | Median 113 | ||
| IQR 51–87 | IQR 83–132 | ||
| Days on ITU | 1.5 ± 3.2 | 2.2 ± 4.2 | <0.001 |
| Median 1.0 | Median 1.0 | ||
| IQR 1.0–1.0 | IQR 1.0–1.0 | ||
| Number of distal anastomosis | NA | 1 (0.5%) | NA |
| 0 | 54 (40%) | ||
| 1 | 29 (22%) | ||
| 2 | 28 (21%) | ||
| 3 | 14 (10%) | ||
| 4 | 8 (5.9%) | ||
| 5 | 1 (0.7%) | ||
| 6 | |||
| Rethoracotomy | 7 (3.6%) | 15 (11%) | 0.007 |
| Cardiopulmonary bypass length (min) | 99 ± 39 | 153 ± 49 | <0.001 |
| Median 102 | Median 151 | ||
| IQR 67–119 | IQR 125–184 | ||
| Prosthetic aortic valve diameter (mm) | 0.020 | ||
| † | |||
| 19 | 5 (2.8%) | 5 (3.9%) | † |
| 21 | 32 (18%) | 27 (21%) | † |
| 23 | 72 (40%) | 62 (49%) | † |
| 25 | 48 (27%) | 26 (21%) | * |
| 27 | 23 (13%) | 7 (5.5%) | |
| Preoperative haemoglobin (mmol/l) | 8.5 ± 0.8 | 8.4 ± 0.9 | 0.317 |
| Median 8.5 | Median 8.4 | ||
| IQR 7.9–9.0 | IQR 7.6–9.0 | ||
| Preoperative platelets (109/l) | 237 ± 63 | 241 ± 93 | 0.487 |
| Median 228 | Median 226 | ||
| IQR 194–228 | IQR 186–264 | ||
| Preoperative leucocytes (109/l) | 7.8 ± 2.4 | 7.9 ± 2.3 | 0.532 |
| Median 7.5 | Median 7.7 | ||
| IQR 6.5–8.5 | IQR 6.4–9.1 | ||
| Preoperative INR | 1.2 ± 0.5 | 1.2 ± 0.6 | |
| Median 1.0 | Median 1.0 | ||
| IQR 1.0–1.1 | IQR 1.0–1.1 | ||
| ESR (mm/h) | 14 ± 1.0 | 18 ± 1.5 | 0.002 |
| Median 9.0 | Median 13.0 | ||
| Q1,Q3 5.0–18 | Q1,Q3 7.0–22 |
*P-value between AVR and AVR + CABG is less than 0.05 for this variable
†P-value between AVR and AVR + CABG is more than 0.05 for this variable
ESR erythrocyte sedimentation rate, INR international normalised ratio, ITU intensive care unit, NA not applicable
Follow-up
Follow-up for up to 120 months was achieved for all patients. Median follow-up was 48 months (IQR 29–76 months). For patients in the AVR-CABG group, median follow-up was 46 months (IQR 26–82 months) and for patients in the AVR group, 49 months (IQR 30–71 months).
The in-hospital and 30-day follow-up mortality was 3.0% in AVR-CABG patients and 2.0% in AVR patients. The expected mortality as estimated from the logistic EuroSCORE was 8.2% for patients in the AVR-CABG group and 6.6% for patients in the AVR group.
Cause of death
Among the total cohort there were 38 (55%) cardiovascular deaths and 31 (45%) non-cardiovascular deaths. Cardiovascular death accounted for 59% of deaths in the AVR-CABG group and for 54% in the AVR group (p-value 0.683). Of the cardiovascular deaths, 15 were caused by fatal arrhythmias, 6 by congestive heart failure, 5 by cerebrovascular accidents, 4 by surgical complications, 3 by endocarditis, 2 by myocardial infarction and 3 by assorted vascular diseases (p-value 0.211). Of the non-cardiovascular deaths, 16 were caused by cancer, 6 by frailty and 9 by other causes e.g. pneumonia, liver cirrhosis, bleeding gastric ulcer.
Kaplan-Meier survival analysis
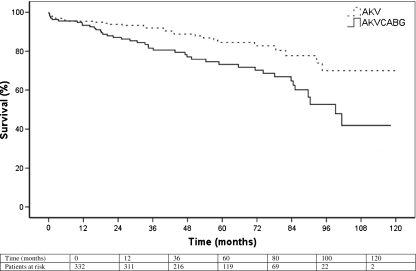
The 5-year survival for the total population was 80% (Table 3, Fig. 1). Survival was relatively similar between groups in the first 12 months with 93% in the AVR-CABG group and 95% in the AVR group. After 12 months, the graphs diverged with 5-year survival being 73% in the AVR-CABG group and 85% in the AVR group. The log-rank tests, comparing survival between the groups, emphasise this trend with no statistically significant difference after 1 year (p = 0.414), but after 3 years (p = 0.009) and 5 years (p = 0.012) the AVR-CABG group had a significantly worse survival than the AVR group.
Table 3.
Kaplan-Meier survival analysis
| 1-year survival with 95% CI | p-value | 3-year survival with 95% CI | p-value | 5 year survival with 95% CI | p-value | |
|---|---|---|---|---|---|---|
| AVR | 95% (0.93–0.98) | 0.414 | 92% (0.88–0.96) | 0.009 | 85% (0.78–0.91) | 0.012 |
| AVR-CABG | 93% (0.89–0.98) | 82% (0.75–0.89) | 73% (0.65–0.82) | |||
| Total population | 95% (0.92–0.98) | 88% (0.84–0.91) | 80% (0.75–0.85) |
Fig. 1.
Kaplan Meier survival curve. Log-rank tests: 1 year; p = 0.414, 3 years; p = 0.009; 5 years; 0.012
Cox proportional hazard analysis
Independent risk factors for all-cause mortality were creatinine value (HR 1.09; CI 1.03–1.15 per 10 units increase), prior CABG (HR 2.5; CI 1.1–5.6) and age (HR 1.06; CI 1.03–1.10) (Table 4). While concomitant CABG did univariately predict for mortality, it did not remain an independent predictor for mortality after multivariate analysis (Table 4).
Table 4.
Association between baseline characteristics and follow-up
| Covariate | Mortality at FU | % | Univariate Cox regression analysis | Multivariate Cox regression analysis | ||
|---|---|---|---|---|---|---|
| Crude HR and 95% CI | P-value crude HR | Adjusted HR and 95% CI | P-value adjusted HR | |||
| AVR | 28/197 | 14 | 1 | 0.003 | 1.34 (0.79–2.26) | 0.267 |
| AVR-CABG | 41/135 | 30 | 2.08 (1.29–3.37) | |||
| Male | 43/207 | 21 | 1.14 (0.70–1.86) | 0.604 | ||
| Female | 26/125 | 21 | 1 | |||
| Age (years)* | 1.07 (1.04–1.11) | <0.001 | 1.06 (1.03–1.10) | <0.001 | ||
| BMI (kg/m2)* | 0.96 (0.90–1.03) | 0.247 | ||||
| Preoperative creatinine (μmol/l) † | 1.10 (1.05–1.16) | <0.001 | 1.09 (1.03–1.15) | 0.004 | ||
| Prior CABG | 7/18 | 39 | 2.64 (1.20–5.80) | 0.003 | 2.47 (1.09–5.61) | 0.030 |
| No prior CABG | 62/314 | 20 | 1 | |||
| Prior MI | 9/23 | 39 | 2.30 (1.14–4.64) | 0.020 | 1.40 (0.66–2.99) | 0.381 |
| No prior MI | 60/309 | 19 | 1 | |||
| Prior PTCA | 7/23 | 30 | 2.23 (1.02–4.91) | 0.045 | 1.24 (0.54–2.88) | 0.613 |
| No prior PTCA | 62/309 | 20 | 1 | |||
| Cardiac family history | 21/82 | 26 | 1.25 (0.74–2.09) | 0.405 | ||
| 48/250 | 19 | 1 | ||||
| No cardiac family history | ||||||
| Prior valve surgery | 2/3 | 67 | 3.39 (0.82–13.91) | 0.091 | ||
| No prior valve surgery | 67/329 | 20 | 1 | |||
| Hypertension | 40/174 | 23 | 1.34 (0.83–2.16) | 0.235 | ||
| No hypertension | 29/158 | 18 | 1 | |||
| Hyperlipidaemia | 24/124 | 19 | 1.05 (0.64–1.72) | 0.863 | ||
| 1 | ||||||
| No hyperlipidaemia | 45/208 | 22 | ||||
| Smoker | 6/43 | 14 | 0.56 (0.24–1.30) | 0.175 | ||
| Non smoker | 63/289 | 22 | 1 | |||
| Angina pectoris | 33/130 | 25 | 1.26 (0.78–2.02) | 0.348 | ||
| No angina pectoris | 36/202 | 18 | 1 | |||
| Diabetes mellitus | 19/55 | 35 | 2.19 (1.29–3.71) | 0.004 | 1.33 (0.75–2.35) | 0.333 |
| No diabetes mellitus | 50/277 | 18 | 1 | |||
| Intermittent claudication | 5/16 | 31 | 1.92 (0.77–4.79) | 0.161 | ||
| 64/316 | 20 | 1 | ||||
| No intermittent claudication | ||||||
| Left main stenosis | 68/332 | 21 | 0.44 (0.06–3.17) | 0.415 | ||
| No left main stenosis | 1/10 | 10 | 1 | |||
| COPD | 10/43 | 23 | 1.36 (0.69–2.67) | 0.387 | ||
| No COPD | 59/289 | 20 | 1 | |||
| Prior TIA/CVA | 2/69 | 2.9 | 2.28 (0.56–9.34) | 0.310 | ||
| No prior TIA/CVA | 3/263 | 1.1 | 1 | |||
| NYHA class ‡ | 1.24 (0.77–2.00) | 0.373 | ||||
| Left ventricle function ‡ | 1.09 (0.84–1.41) | 0.522 | ||||
| Urgency ‡ | 1.15 (0.83–1.60) | 0.400 | ||||
* HR for every unit increase, † HR for every 10 units increase, ‡ HR for every increase in class
AVR aortic valve replacement, BMI body mass index, CABG coronary artery bypass grafting, COPD chronic obstructive pulmonary disease, CVA cerebrovascular accident, MI myocardial infarction, NYHA New York Heart Association, PTCA percutaneous transluminary coronary angiography, TIA transient ischaemic attack
Discussion
We report the outcomes and predictors for mortality in consecutive, all-comer patients who underwent isolated AVR or concomitant AVR and CABG. An increased creatinine value, older age and previous CABG predicted a decreased survival, while concomitant CABG was not an independent risk factor. Furthermore, the EuroSCORE significantly overestimated the 30-day and in-hospital survival in this consecutive series of patients. Finally, 5-year survival was significantly better in the isolated AVR group compared with the concomitant AVR and CABG group (85 vs 73%; p = 0.012).
Survival estimates
The expected operative mortality based on the logistic EuroSCORE was two- to threefold higher than the observed mortality. Multiple reports from different countries have demonstrated the EuroSCORE to significantly overestimate operative mortality for AVR [10–13]. With this study, we confirm that the EuroSCORE overestimates operative mortality for AVR in the Dutch population as well. Brown et al. postulate that this overestimation may be caused by the EuroSCORE only distinguishing between isolated CABG and procedures other than CABG. As a consequence, the relatively lower risk procedures, such as isolated AVR, result in the same EuroSCORE as the relatively higher risk procedures, such as a multiple valve replacement with concomitant CABG [3, 11].
The overall 5-year survival in our population was 80%. Both Chikwe et al. and Kvidal et al. examined the outcome after AVR in unselected populations with comparable baseline characteristics and reported 5-year survival rates comparable with ours. Chikwe et al. reported a 5-year survival of 80%, while Kvidal et al. reported 83% [7, 14]. It must be noted, however, that Kvidal et al. did not take the first 30 days after surgery into account. As these two studies demonstrate almost identical 5-year survival rates to our own results, we confirm that the 5-year survival rates for isolated AVR (85%) and concomitant AVR-CABG (73%) are reliable estimates for clinical use.
Risk prediction
The variables that independently predicted for mortality were an older age, prior CABG and higher creatinine values. These predictors are in accordance with existing evidence. Both Collins et al. and Fighali et al. have confirmed that previous CABG predicts a worse long-term outcome after AVR [15, 16]. Florath et al. describe a higher creatinine value to predict a worse 6-month survival after AVR in octogenarians [17]. Our study demonstrates that the detrimental effect of higher creatinine values is extended beyond 6 months and not only in octogenarians but in the general population as well.
Survival for patients receiving isolated AVR was better than survival for patients receiving concomitant AVR-CABG. In the multivariate analysis, however, concomitant CABG did not remain an independent risk factor for mortality with a hazard ratio of 1.3 (CI 0.8–2.3). This discrepancy can be explained by the difference in clinical risk profiles between the AVR group and the AVR-CABG group. Patients in the AVR-CABG group were older, had a worse kidney function (expressed by a higher creatinine value) and had received prior CABG more often. He et al. theorise that coronary artery disease in the patients receiving concomitant CABG could increase their risk of mortality even after surgery. Our study does not support this claim as we found an equal proportion of cardiovascular deaths in the AVR group and the AVR-CABG group.
It is noteworthy that Kvidal et al. reported the same hazard ratio of 1.3 (CI 1.0–1.7) for concomitant CABG. However, contrary to our findings, concomitant CABG did independently predict for mortality in their analysis. When comparing the outcomes, it is important to note that in their multivariate analysis, Kvidal et al. did not take the creatinine value and prior CABG into account. Had they done so, it is possible that concomitant CABG would not remain independently associated with decreased survival.
Limitations
The current results are derived from a single centre, so their applicability to other populations is uncertain. Nevertheless baseline and clinical outcome parameters are comparable with other studies investigating AVR, indicating our results are likely to be applicable to other populations [1, 4, 6, 7].
Unfortunately, data on whether the aortic valve pathology was regurgitation, stenosis or mixed were unavailable. Both Melby et al. and Jones et al. found regurgitating aortic valve disease to be an independent risk factor. Including the type of aortic valve pathology as an additional variable might have improved the accuracy of our analysis [6, 8], and this is also true for preoperative atrial fibrillation, as Kvidal et al. found this to be an independent risk factor for mortality in their study [7].
Conclusion
Unselected, consecutive patients who underwent aortic valve replacement surgery and who received concomitant bypass surgery between 2001–2010 had higher 5-year mortality than patients receiving isolated AVR. Prior CABG, renal function, age but not concomitant CABG remained independently associated with increased mortality. Finally, the observed mortality in this unselected patient group compared favourably with preoperative risk assessment using the EuroSCORE risk model.
References
- 1.Shahian DM, O'Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1–coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S2–S22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2–isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl):S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 3.Shahian DM, O’Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3–valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S43–S62. doi: 10.1016/j.athoracsur.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 4.Ambler G, Omar RZ, Royston P, et al. Generic, simple risk stratification model for heart valve surgery. Circulation. 2005;112(2):224–231. doi: 10.1161/CIRCULATIONAHA.104.515049. [DOI] [PubMed] [Google Scholar]
- 5.Roques F, Michel P, Goldstone AR, et al. The logistic EuroSCORE. Eur Heart J. 2003;24(9):881–882. doi: 10.1016/S0195-668X(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 6.Jones JM, Lovell D, Cran GW, et al. Impact of coronary artery bypass grafting on survival after aortic valve replacement. Interact Cardiovasc Thorac Surg. 2006;5(3):327–330. doi: 10.1510/icvts.2005.118349. [DOI] [PubMed] [Google Scholar]
- 7.Kvidal P, Bergstrom R, Horte LG, et al. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000;35(3):747–756. doi: 10.1016/S0735-1097(99)00584-7. [DOI] [PubMed] [Google Scholar]
- 8.Melby SJ, Zierer A, Kaiser SP, et al. Aortic valve replacement in octogenarians: risk factors for early and late mortality. Ann Thorac Surg. 2007;83(5):1651–1656. doi: 10.1016/j.athoracsur.2006.09.068. [DOI] [PubMed] [Google Scholar]
- 9.He GW, Grunkemeier GL, Starr A. Aortic valve replacement in elderly patients: influence of concomitant coronary grafting on late survival. Ann Thorac Surg. 1996;61(6):1746–1751. doi: 10.1016/0003-4975(96)00143-9. [DOI] [PubMed] [Google Scholar]
- 10.Grossi EA, Schwartz CF, Yu PJ, et al. High-risk aortic valve replacement: are the outcomes as bad as predicted? Ann Thorac Surg. 2008;85(1):102–106. doi: 10.1016/j.athoracsur.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Brown ML, Schaff HV, Sarano ME, et al. Is the European System for Cardiac Operative Risk Evaluation model valid for estimating the operative risk of patients considered for percutaneous aortic valve replacement? J Thorac Cardiovasc Surg. 2008;136(3):566–571. doi: 10.1016/j.jtcvs.2007.10.091. [DOI] [PubMed] [Google Scholar]
- 12.Gummert JF, Funkat A, Osswald B, et al. EuroSCORE overestimates the risk of cardiac surgery: results from the national registry of the German Society of Thoracic and Cardiovascular Surgery. Clin Res Cardiol. 2009;98(6):363–369. doi: 10.1007/s00392-009-0010-8. [DOI] [PubMed] [Google Scholar]
- 13.Bhatti F, Grayson AD, Grotte G, et al. The logistic EuroSCORE in cardiac surgery: how well does it predict operative risk? Heart. 2006;92(12):1817–1820. doi: 10.1136/hrt.2005.083204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chikwe J, Croft LB, Goldstone AB, et al. Comparison of the results of aortic valve replacement with or without concomitant coronary artery bypass grafting in patients with left ventricular ejection fraction < or =30% versus patients with ejection fraction >30% Am J Cardiol. 2009;104(12):1717–1721. doi: 10.1016/j.amjcard.2009.07.059. [DOI] [PubMed] [Google Scholar]
- 15.Collins JJ, Jr, Aranki SF. Management of mild aortic stenosis during coronary artery bypass graft surgery. J Card Surg. 1994;9(2 Suppl):145–147. doi: 10.1111/j.1540-8191.1994.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 16.Fighali SF, Avendano A, Elayda MA, et al. Early and late mortality of patients undergoing aortic valve replacement after previous coronary artery bypass graft surgery. Circulation. 1995;92(9 Suppl):II163–II168. doi: 10.1161/01.cir.92.9.163. [DOI] [PubMed] [Google Scholar]
- 17.Florath I, Albert A, Boening A, et al. Aortic valve replacement in octogenarians: identification of high-risk patients. Eur J Cardiothorac Surg. 2010;37(6):1304–1310. doi: 10.1016/j.ejcts.2009.12.025. [DOI] [PubMed] [Google Scholar]