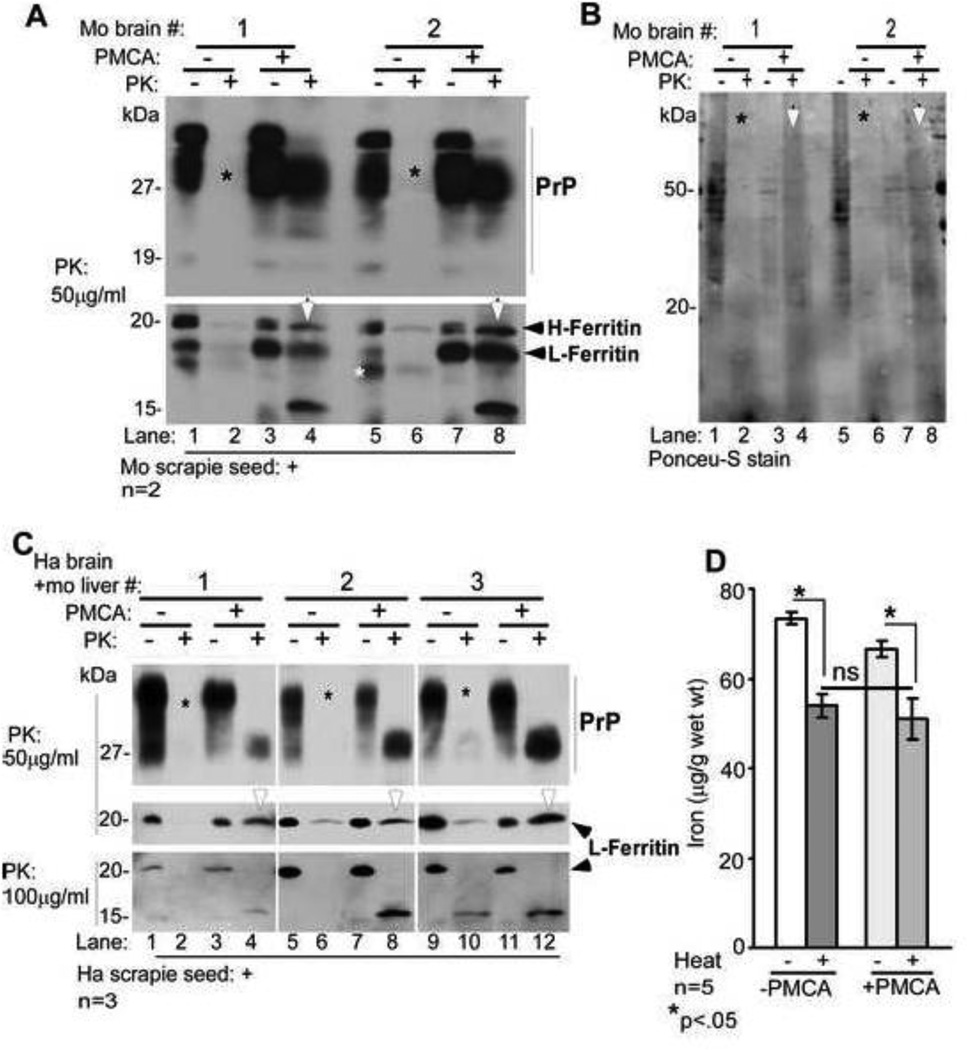
Figure 5. Amplification of PrPSc in vitro does not increase the heat stable pool of iron.
(A) Normal mouse brain homogenate seeded with mouse scrapie was either set aside (−PMCA) or subjected to PMCA (+PMCA). Samples were treated with buffer (−PK) or PK (+PK) and subjected to Western blotting. Probing for PrP reveals the expected glycoforms in −PK samples (lanes 1, 3, 5, & 7). Treatment with PK causes complete degradation of PrP in −PMCA samples (lanes 2, & 6, *), and typical PK-resistant C-terminal fragments of PrPSc in +PMCA samples (lanes 4 & 8). Re-probing for ferritin shows similar pattern as PrP. Both isoforms of ferritin (H- and L-chain) are detected in −PK samples (lanes 1, 3, 5, & 7). PK treatment degrades both isoforms of ferritin in −PMCA samples (lanes 2 & 6), while +PMCA samples resist PK and show a faster migrating 15kDa form (lanes 4 & 8). A representative blot from five separate experiments is shown. (B) Visualization of transferred proteins by Ponceu-S stain shows an overall decrease in +PMCA samples (lanes 3, 4, 7, & 8, *), and increased PK resistance of several proteins in PMCA samples (lanes 4 & 8 vs. 2 & 6, white arrow-heads). (C) Similar evaluation of hamster brain and mouse liver homogenates seeded with hamster scrapie reveals PK-resistant PrPSc and ferritin in +PMCA samples (lanes 4, 8, & 12), not in −PMCA samples (lanes 2, 6, & 10). Increase in the concentration of PK reveals a faster migrating PK resistant fragment of ferritin (lanes 4, 8, & 12). (D) Iron associated with methanol precipitated protein pellet from −PMCA and +PMCA samples decreases significantly after boiling as expected. However, there is no difference in the iron content of boiled −PMCA and +PMCA samples despite successful amplification of PrPSc. A and C are representative blots of 3 different experiments. n=5. *p<0.05.