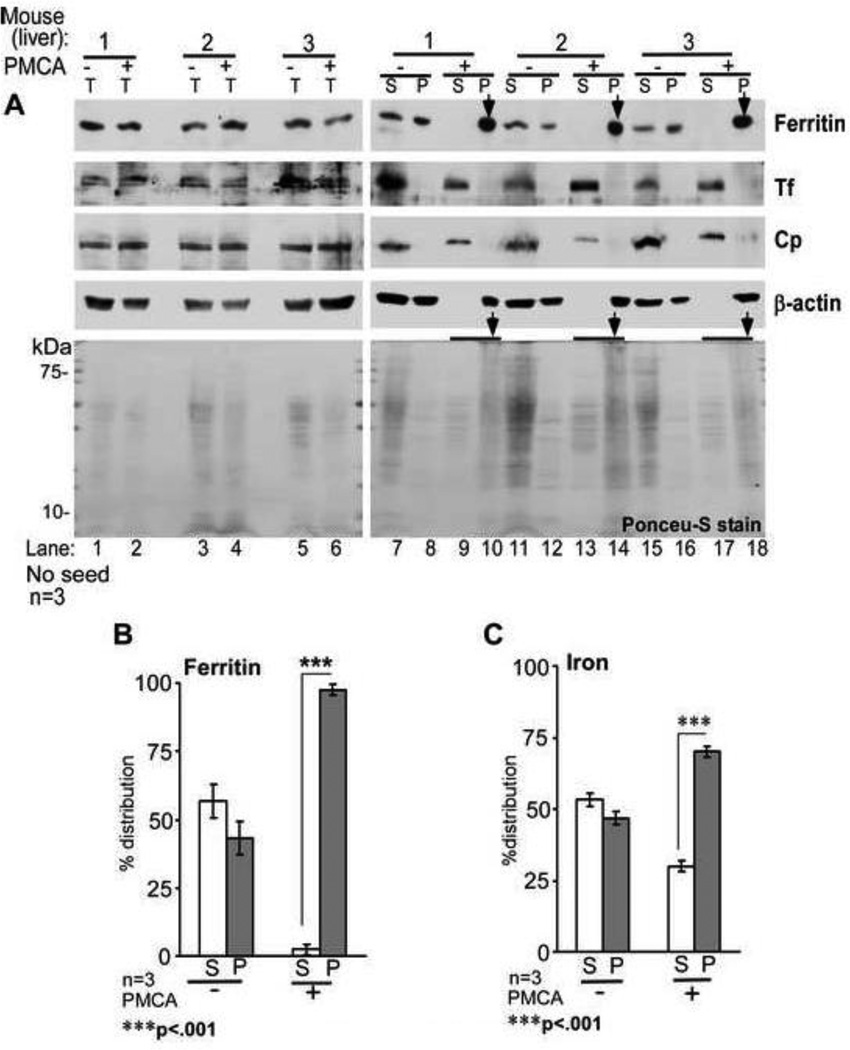
Figure 6. PMCA reaction induces aggregation of ferritin in liver homogenates.
(A) Normal mouse liver homogenates subjected to PMCA (+PMCA) or not (−PMCA) were centrifuged to separate detergent soluble (S) and insoluble (P) fractions, and analyzed by Western blotting. Probing of total (T) samples before centrifugation reveals equal distribution of ferritin in −PMCA and +PMCA samples (lanes 1–6). Following centrifugation, ferritin partitions equally between S and P fractions in −PMCA samples (lanes 7, 8, 11, 12, 15, & 16), but shifts almost completely to the P fraction following PMCA (lanes 10, 14, & 18, *). Unlike ferritin, Tf and Cp remain in the S fraction even after PMCA (lanes 7, 9, 11, 13, 15, & 17). Surprisingly, β-actin also aggregates by PMCA (lanes 10, 14, & 18). Ponceu-S staining of all proteins reveals slight degradation by PMCA (lanes 2, 4, & 6) and PK resistance of several proteins in the P fraction (lanes 10, 14, & 18). A representative blot of 3 different experiments is shown. (B) Percent distribution of ferritin shows 97.5% in the P fraction following PMCA. Since β-actin aggregates by PMCA, lanes 1–6 were used as loading controls for lanes 7–18. n=3. ***p<.001. (C) Protein-associated iron shows similar distribution as ferritin. Non-correspondence of ferritin and iron in the S fraction of +PMCA samples is due to contribution from low molecular weight iron compounds and soluble iron rich proteins such as Tf. n=3. ***p<.001.