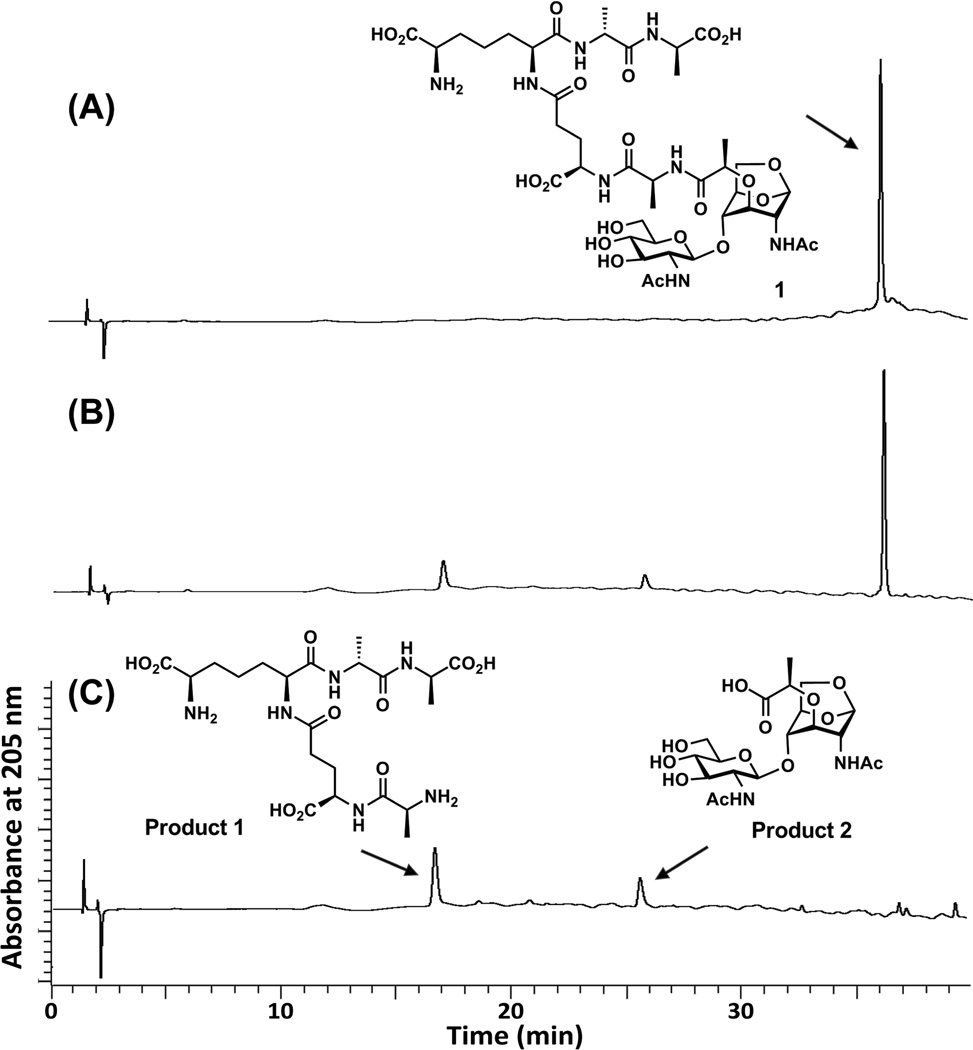
Figure 2.
The AmpD reaction with compound 1. (A) The single peak is compound 1 at time zero. (B) Compound 1 was hydrolyzed by AmpD, and the two products were formed (retention time at 16.5 min and 25.5 min) at 60 min of incubation. (C) The AmpD reaction mixture at 3 h. The two new peaks correspond to product 1 (pentapeptide) and product 2 (GlcNAc-anhMurNAc). The reaction was monitored on a C18 reversed-phase HPLC column (Symmetry Shield RP18, 5 µm, 3.9 mm by 150 mm; Waters) on a PerkinElmer series 200 System. The column was equilibrated with 0.05% trifluoroacetic acid in water and eluted with a linear acetonitrile gradient from 0 to 15% over 40 min with a flow rate of 1 mL/min. The column effluent was monitored at 205 nm.