Abstract
Objective
To seek evidence for causative secondary changes in extraocular muscle volume, cross-sectional area, and contractility in superior oblique (SO) palsy using magnetic resonance imaging, given that vertical deviations in SO palsy greatly exceed those explained by loss of SO vertical action alone.
Methods
High-resolution, quasi-coronal orbital magnetic resonance images in target-controlled central gaze, supraduction, and infraduction were obtained in 12 patients with chronic unilateral SO palsy and 36 age-matched healthy volunteers using an 8-cm field of view and 2-mm slice thickness. Digital image analysis was used to quantify rectus extraocular muscle and SO cross-sectional areas and volumes. Measurements were compared with those of controls in central gaze to detect hypertrophy or atrophy and during vertical gaze changes to detect excess contractility.
Results
In central gaze, the paretic SO was significantly atrophic (P<.001) and the contralesional superior rectus (SR) was significantly hypertrophic (P=.02). Across the range of vertical duction from supraduction to infraduction, both the contralesional SR (P=.04) and inferior rectus (P=.001) exhibited significantly supernormal contractile changes in maximum cross-sectional area. Contractile changes in the ipsilesional SR and inferior rectus exhibited a similar but insignificant trend (.08<P<.12).
Conclusions
Central gaze hypertrophy of the contralesional SR may be secondary to chronic excess innervation to compensate for relative hypotropia of this eye. Supernormal contralesional SR and inferior rectus contractility suggests that dynamic patterns of abnormal innervation to vertical rectus extraocular muscles may contribute to large hypertropias often observed in SO palsy.
Superior oblique (SO) palsy is a common cause of vertical strabismus. However, both the SO and inferior oblique (IO) muscles contribute little to vertical duction in healthy subjects.1–6 This raises the fundamental question of how a palsy of the SO, an extraocular muscle (EOM) whose primary function is intorsion,7 can create a large hypertropia.
Studies in rhesus monkeys suggest that SO palsy alone is not sufficient to create a large vertical strabismus.8 After experimental trochlear neurectomy to induce SO palsy in monkeys, hypertropia slowly diminished under monocular conditions but greatly increased when binocular vision was permitted.8 This study suggests that abnormal neural input during binocular viewing was driving the large vertical deviation in experimental SO palsy in the rhesus monkey.
It is unclear whether the same type of abnormal innervation occurs in humans with SO palsy. Despite a wealth of observational data collected over many years, the mechanisms that create incomitant vertical strabismus in SO palsy are poorly understood. Part of the confusion probably stems from poor specificity of the clinical examination in detecting isolated SO palsies.9 The 3-step test in particular has been the cornerstone for the diagnosis and classification of cyclovertical strabismus for generations of clinicians, but it poorly distinguishes among chronic unilateral SO palsy, bilateral SO palsies,10 heterotopicrectus EOM pulleys,11 or other orbital or structural EOM abnormalities.9 If traditional teaching were to hold true, then the most common procedure for SO palsy, IO weakening, should increase the head tilt–dependent change in hypertropia; instead, the opposite has been demonstrated.12 Studies that use only the clinical examination to determine the presence of SO palsy inevitably include a heterogeneous group of causes for incomitant vertical strabismus.
Additional confusion is created by the common clinical vernacular that implicates EOM “overaction” and “underaction” when excessive or reduced ductions into tertiary fields of gaze are observed.7 Those misleading terms imply a primary problem of a specific EOM when in fact there are many other potential causes of the abnormal eye movement.13 Excessive elevation in adduction, for example, commonly described as IO overaction, can be caused by aberrant innervation, orbital dystopy, and EOM pulley abnormalities in addition to overcontraction of an IO.11,13
Three potential causes have been postulated for primary EOM overaction: hypertrophy, excessive innervation, or change in fiber type (analogous to replacing slow-twitch with fast-twitch muscle fibers) with a resultant change in the EOM’s response to a given level of innervation.13 The last type of intrinsic muscular change has been demonstrated in skeletal muscle in animals14 but not in EOMs.13 The purpose of this study was to determine whether high-resolution magnetic resonance imaging (MRI) can detect primary EOM overaction, either from hypertrophy or excess innervation, in patients with SO palsy.
METHODS
This study was conducted in 36 healthy, orthotropic paid volunteers who were recruited by advertisement and 12 subjects with chronic unilateral SO palsy confirmed by both clinical examination and the presence of significant SO atrophy on MRI,15,16 with no history of strabismus surgery. Each subject gave written informed consent according to a protocol conforming to the Declaration of Helsinki and approved by the Human Subject Protection Committee at the University of California, Los Angeles. Data collection was compliant with the Health Insurance Portability and Accountability Act of 1996.
Healthy subjects underwent a comprehensive eye examination to verify normal visual acuity, normal ocular motility, stereoacuity, and ocular anatomy. Subjects with SO palsy underwent the same comprehensive eye examination as well as a Hess screen test.
Each subject underwent high-resolution T1-weighted or T2-weighted fast spin-echo MRI with surface coils and a 1.5-T Signa scanner (General Electric, Milwaukee, Wisconsin) using protocols described in detail elsewhere.17,18 Images were obtained in a quasi-coronal fashion (Figure 1) in a matrix of 256×256 pixels over an 8-cm field of view (313-μm pixel resolution) with 2-mm slice thickness. Images were obtained by scanning the fixating eye in central gaze in all subjects and in supraduction and infraduction for most subjects, with imaging in secondary gaze positions limited in some subjects by fatigue during image acquisition.
Figure 1.
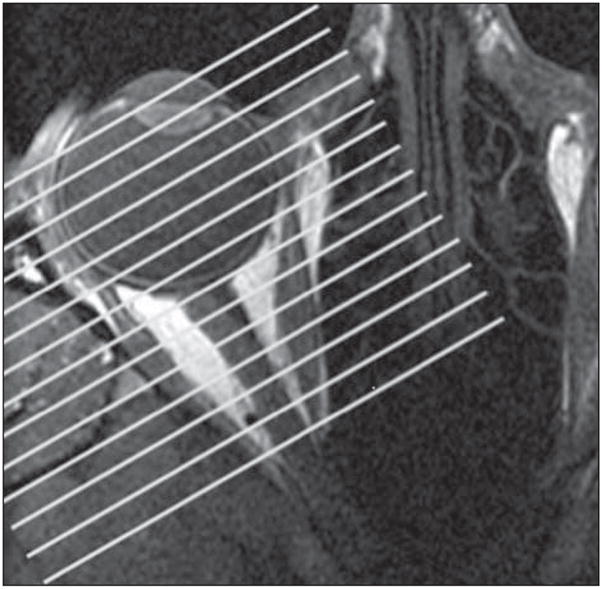
Axial magnetic resonance image of a right orbit demonstrating by parallel white lines the planes of quasi-coronal images perpendicular to the long axis of the orbit.
Digital MRIs were quantified using the program ImageJ 1.37v (W. Rasband, National Institutes of Health, Bethesda, Mary-land). For the 6 contiguous image planes beginning at the globe–optic nerve junction and extending 12 mm posteriorly, each rectus EOM and the SO were manually outlined (Figure 2) and the cross-sectional area was obtained using the Area function of ImageJ. Volumes were determined by multiplying the cross-sectional areas by the 2-mm slice thickness and summing the volumes for all 6 image planes.
Figure 2.
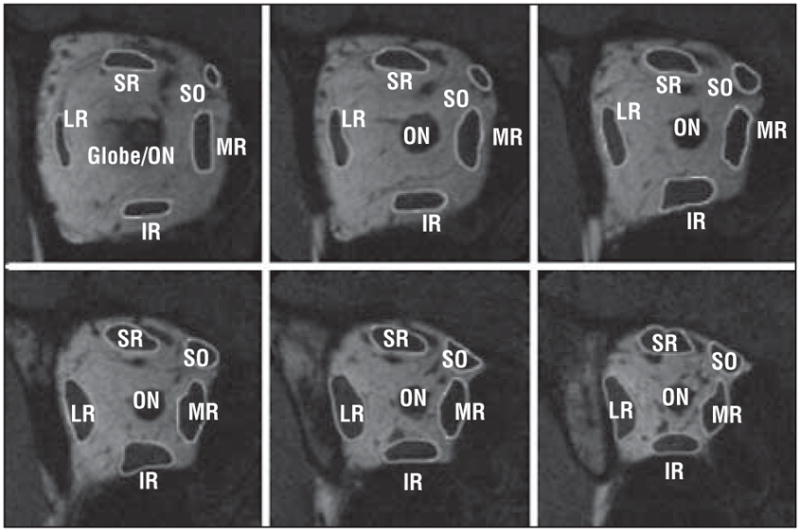
Quasi-coronal magnetic resonance images of a normal right orbit. Each rectus extraocular muscle and the superior oblique (SO) were manually outlined using the program ImageJ 1.37v (W. Rasband, National Institutes of Health, Bethesda, Maryland) to determine the cross-sectional area for the 6 contiguous image planes contiguously posterior to the globe–optic nerve (ON) junction. SR indicates superior rectus; MR, medial rectus; IR, inferior rectus; and LR, lateral rectus.
The EOM volumes and maximum cross-sectional areas in central gaze were considered measures of hypertrophy. The change in EOM maximum cross-sectional area from supra-duction to infraduction was considered a measure of contractility. Statistical comparisons were made using paired t tests.
RESULTS
The mean ages for the subjects with SO palsy (33.3 years) and the healthy subjects (36.6 years) were equivalent (P=.47). The clinical data for the subjects with SO palsy were, by design, very homogeneous—each case had a previously unoperated unilateral SO palsy present for a minimum of 6 months (mean, 11.7 years; range, 6 months to 43 years). The incomitant strabismus was, on average, typical for unilateral SO palsy. Using a clinical scale representing a normal value of 0 to an imbalance of ±4, the mean (SD) underdepression in adduction was −2.0 (1.0) and the mean overelevation in adduction was 1.8 (1.1). The mean primary gaze hypertropia was 9.5 (7.0) prism diopters (Δ), increasing to 23.1 (11.7) Δ on adduction of the affected eye and decreasing to 5.8 (6.3) Δ on abduction of the affected eye (Table 1). On average, the hypertropia was 14.6 Δ greater in ipsilesional than contralesional head tilt.
Table 1.
Clinical Data for Subjects With Superior Oblique Palsy
| Patient No./Sex/Age, ya | Hypertropia, Prism Diopters
|
||
|---|---|---|---|
| Central Gaze | Adductionb | Abductionb | |
| 1/F/15 | 12 | 20 | 6 |
| 2/M/47 | 18 | 12 | 18 |
| 3/M/28 | 14 | 25 | −3 |
| 4/M/14 | 4.5 | 20 | 0 |
| 5/M/42 | 12 | 10 | 8 |
| 6/M/16 | 2 | 30 | 4 |
| 7/M/27 | 8 | 10 | 0 |
| 8/M/39 | 4 | 40 | 4 |
| 9/M/70 | 8 | 12 | 8 |
| 10/F/38 | 2 | Not done | Not done |
| 12/F/46 | 25 | 40 | 15 |
| 13/F/18 | 5 | 35 | 4 |
| Mean | 9.5 (2.0) | 23.1 (2.1) | 5.8 (1.9) |
The mean (SD) age was 33.3 (4.9) years.
Adduction and abduction refer to the position of the eye with superior oblique palsy.
All 72 orbits in the 36 healthy subjects and 24 orbits in the 12 subjects with SO palsy were imaged in central gaze. In subjects with SO palsy, we separately analyzed the orbit ipsilesional to the palsied SO and the uninvolved, contralesional orbit.
In central gaze, only 2 EOMs had significantly abnormal volumes (Table 2) and maximum cross-sectional areas (Table 3) in subjects with SO palsy. The ipsilesional, paretic SO had approximately 50% of normal volume (96.8 vs 176.4 mm3, respectively; P<.001) and maximum cross-sectional area (10.3 vs 19.2 mm2, respectively; P<.001), an anticipated result since visible SO atrophy on MRI was a selection criterion for inclusion in the study. However, the contralesional superior rectus (SR) was significantly hypertrophic compared with that in healthy subjects, having more than 10% greater volume (344.7 vs 307.5 mm3, respectively; P=.02) and maximum cross-sectional area (34.2 vs 30.5 mm2, respectively; P=.02).
Table 2.
Central Gaze Extraocular Muscle Volume
| Orbit | Central Gaze Extraocular Muscle Volume, Mean (SD), mm3 |
||||
|---|---|---|---|---|---|
| SO | MR | SR | LR | IR | |
| Healthy | 176.4 (3.3) | 367.9 (5.8) | 307.5 (5.7) | 345.6 (5.9) | 302.8 (6.3) |
| SO palsy | 96.8 (8.4)a | 377.8 (11.1) | 325.7 (13.1) | 374.7 (20.0) | 318.5 (36.2) |
| SO fellowb | 184.8 (7.5) | 384.2 (12.0) | 344.7 (13.5)a | 368.5 (20.9) | 317.3 (10.5) |
Abbreviations: IR, inferior rectus; LR, lateral rectus; MR, medial rectus; SO, superior oblique; SR, superior rectus.
Statistically significantly different from the healthy orbit.
The SO fellow orbit is the contralateral unaffected orbit. Note hypertrophy of the contralesional SR muscle.
Table 3.
Central Gaze Extraocular Muscle Maximum Cross-sectional Area
| Orbit | Central Gaze Extraocular Muscle Maximum Cross-sectional Area, Mean (SD), mm2 |
||||
|---|---|---|---|---|---|
| SO | MR | SR | LR | IR | |
| Healthy | 19.2 (0.4) | 35.2 (0.6) | 30.5 (0.6) | 37.7 (0.6) | 32.0 (0.7) |
| SO palsy | 10.3 (0.9)a | 37.7 (1.4) | 32.5 (1.2) | 39.5 (2.0) | 32.5 (1.8) |
| SO fellowb | 20.3 (0.7) | 36.6 (1.3) | 34.2 (1.4)a | 40.3 (1.8) | 32.9 (1.6) |
Abbreviations: IR, inferior rectus; LR, lateral rectus; MR, medial rectus; SO, superior oblique; SR, superior rectus.
Statistically significantly different from the healthy orbit.
The SO fellow orbit is the contralateral unaffected orbit. Note hypertrophy of the contralesional SR muscle.
For measurements in supraduction and infraduction, 41 orbits in 25 healthy subjects were compared with 10 contralesional and 7 ipsilesional orbits in subjects with SO palsy, with data collection in these eccentric gaze positions limited in some patients secondary to fatigue during MRI. No changes in EOM volumes from supraduction to infraduction were significantly different from normal (data not shown). Two EOMs, however, had a significantly greater than normal change in maximum cross-sectional area across the range of vertical duction (Table 4). Across the range of vertical duction from infraduction to supraduction, the contralesional SR had a more than 30% greater than normal change in maximum cross-sectional area (13.8 vs 10.3 mm2; respectively; P=.04), while the contralesional inferior rectus (IR) had a more than 80% greater than normal change in maximum cross-sectional area (10.9 vs 6.0 mm2, respectively; P=.001). The ipsilesional SR and IR had similarly larger than normal contractile changes, although that result did not achieve statistical significance (P=.12 and P=.08, respectively). Both the SO and horizontal rectus EOMs exhibited minimal contractile changes during vertical duction (Figure 3).
Table 4.
Vertical Duction Change in Maximum Cross-sectional Areaa
| Orbit | Vertical Duction Change in Maximum Cross-sectional Area, mm2 |
||||
|---|---|---|---|---|---|
| SO | MR | SR | LR | IR | |
| Healthy | −2.3 (0.5) | 0.7 (0.4) | 10.3 (0.8) | 1.3 (0.7) | −6.0 (0.7) |
| SO palsy | −0.4 (0.6) | 0.7 (1.1) | 13.4 (1.8) | 1.2 (1.5) | −9.2 (2.1) |
| SO fellowb | −1.9 (1.5) | 1.3 (1.3) | 13.8 (1.4)c | −0.5 (1.4) | −10.9 (1.0)c |
Abbreviations: IR, inferior rectus; LR, lateral rectus; MR, medial rectus; SO, superior oblique; SR, superior rectus.
Positive values indicate larger cross-sectional areas in supraduction; negative values, larger cross-sectional areas in infraduction.
The SO fellow orbit is the contralateral unaffected orbit.
Statistically significantly different from the healthy orbit.
Figure 3.
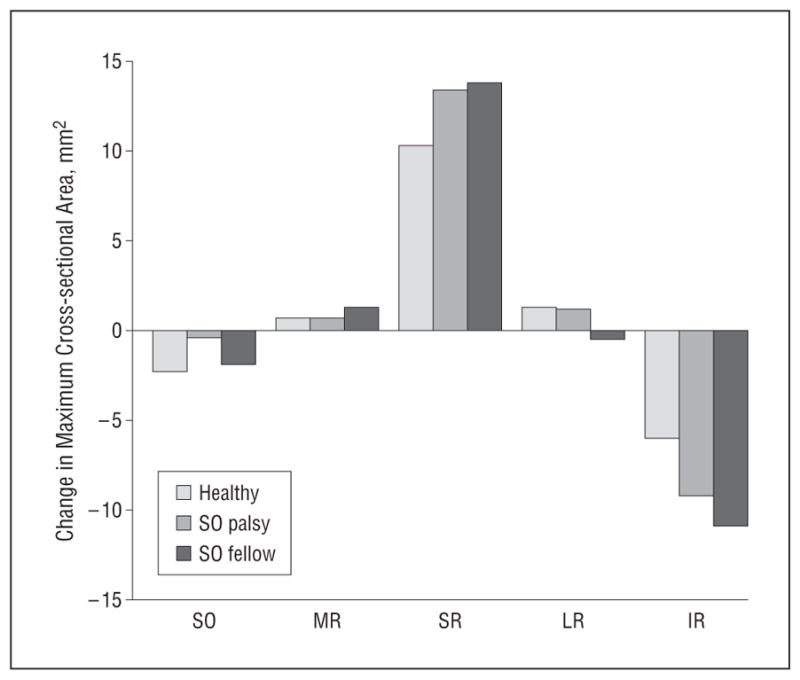
Change in maximum cross-sectional area from infraduction to supraduction. The superior oblique (SO), medial rectus (MR), and lateral rectus (LR) muscles show little change in maximum cross-sectional area, while the vertical rectus muscles in the orbit contralateral to the SO palsy demonstrate excessive contractile change. SO palsy indicates affected orbit; SO fellow, contralateral unaffected orbit; SR, superior rectus; and IR, inferior rectus.
COMMENT
These MRI data provide evidence for 2 types of EOM overaction, hypertrophy and excess innervation. Contralesional SR hypertrophy in SO palsy is a novel finding that cannot be a trivial artifact of eye position or primary vs secondary deviation, since each orbit was imaged with the scanned eye fixating a target in the same position. Inflammatory and infiltrative diseases can enlarge EOMs,19 but none of these subjects had evidence of such disorders. Instead, contralesional SR hypertrophy is most likely secondary to chronic excess innervation to compensate for the relative hypotropia in this eye. This hypertrophic change of the contralesional SR would reduce, not increase, the hypertropia and thus fails to explain why these subjects develop a large vertical deviation.
Excess innervation to the contralesional vertical rectus EOMs does provide a potential explanation for the creation of a large vertical deviation. For the same change in gaze from target-fixated supraduction to target-fixated infraduction, subjects with SO palsy demonstrated a significantly supernormal change in maximum cross-sectional area of the vertical rectus EOMs. The contralesional IR in particular was not hypertrophic in central gaze but had almost twice the normal contractile change in cross-section during vertical duction. This finding confirms and extends the results of an early MRI study of the IR in SO palsy, which also found increased contralesional IR contractility using a different method of analysis.20 This dramatically excessive contractility might plausibly account for the large hypertropia observed in SO palsy because excess contralesional IR innervation in a setting of vertical binocular misalignment would increase the relative hypotropia of this eye. This excess innervation may explain the finding in experimental SO palsy that abnormal innervation under binocular viewing conditions is responsible for increasing the vertical deviation over time.8
This study supports a very limited role for the SO in normal vertical duction.2–6 While these subjects were not imaged performing vertical gaze changes in adduction, the normal SO demonstrated only a small change in maximum cross-sectional area during vertical ductions from central gaze. Interpreting this study in the context of an earlier MRI study that failed to demonstrate excess IO hypertrophy or contractility in SO palsy,18 it becomes difficult to escape the conclusion that the vertical rectus EOMs, the primary supraductors and infraductors, are most likely responsible for dysmotility in SO palsy.3
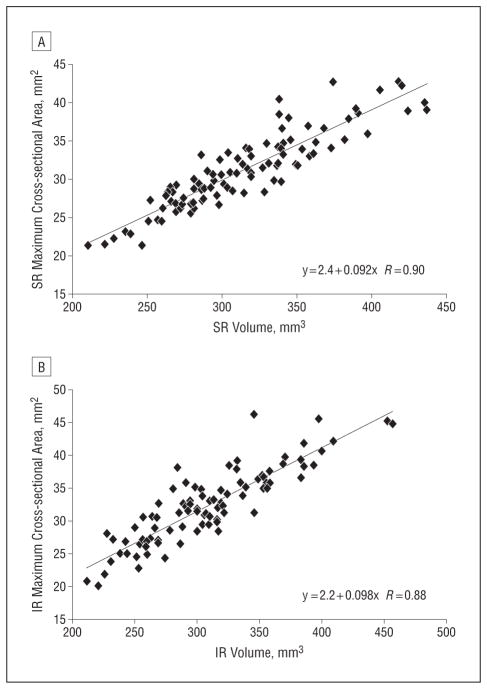
These data suggest that maximum EOM cross-sectional area is the most important functional anatomical indicator of EOM function. The EOM volume is conserved during gaze changes. During contraction and relaxation, an EOM belly can locally thicken and shift mass anteriorly and posteriorly, but the overall volume does not change significantly. Maximum cross-sectional area, however, does change substantially with duction and can provide a metric for either excess or reduced contractility. It also can be used as a surrogate to indicate hypertrophic changes in central gaze because maximum cross-sectional area correlated well with EOM volume (Figure 4). Tight control of gaze is required during imaging, however, to prevent the normal changes in maximum cross-sectional area that occur in eccentric gaze from confounding interpretation.
Figure 4.
Maximum cross-sectional area for all orbits, both those with superior oblique palsy and those that were healthy, showing linear correlation with superior rectus (SR) volume (R=0.90) (A) and linear correlation with inferior rectus (IR) volume (R=0.88) (B).
This study cannot resolve all controversies concerning EOM overaction. Magnetic resonance imaging cannot detect hypothesized intrinsic changes within the EOM that might affect contractility, such as fiber type changes, sarcomere addition, or fibrosis and contracture. Changes in sarcomere size have been shown to occur in EOMs21 and have been speculated to affect the EOMs’ mechanical behavior in a fashion similar to overaction.13 However, such changes have not been demonstrated in association with cyclovertical strabismus in humans or animals. This study also does not explain why excessive contractile changes occur in other EOMs in SO palsy or what neurological and/or sensory mechanisms might be responsible for the presumed abnormal innervation.
In conclusion, this study provides evidence for 2 ways in which the vertical rectus EOMs overact in the orbit contralesional to SO palsy. Static hypertrophy of the contralesional SR, as demonstrated in central gaze, might plausibly be a compensatory mechanism to reduce the relative hypotropia of that eye, but the supernormal contractile changes, particularly in the contralesional IR, would increase the vertical deviation and cannot be regarded as compensatory. Dynamic, not static, changes in the pattern of EOM innervation presumably create the large hypertropias observed in SO palsy. It seems unlikely that common operations treating SO palsy, such as IO weakening, could work by merely reversing mechanical changes due to the palsy. It remains to be seen whether surgical treatment of SO palsy induces beneficial dynamic changes in abnormal rectus EOM innervation.
Acknowledgments
Funding/Support: This study was supported by grant EY08313 from the National Eye Institute. Dr Demer is supported by a Lilly Disney Award for Amblyopia Research from Research to Prevent Blindness.
Role of the Sponsor: The National Eye Institute funded the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosure: None reported.
Disclaimer: Magnetic resonance imaging surface coils used in this project are not approved by the US Food and Drug Administration for this purpose. The authors have no proprietary interests in the surface coils.
Previous Presentation: This paper was presented at the 36th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus; April 15, 2010; Orlando, Florida.
References
- 1.Boeder P. The co-operation of extraocular muscles. Am J Ophthalmol. 1961;51:469–481. [Google Scholar]
- 2.Robinson DA. A quantitative analysis of extraocular muscle cooperation and squint. Invest Ophthalmol Vis Sci. 1975;14(11):801–825. [PubMed] [Google Scholar]
- 3.Robinson DA. Bielschowsky head-tilt test, II: quantitative mechanics of the Bielschowsky head-tilt test. Vision Res. 1985;25(12):1983–1988. doi: 10.1016/0042-6989(85)90023-9. [DOI] [PubMed] [Google Scholar]
- 4.Miller JM, Robinson DA. A model of the mechanics of binocular alignment. Comput Biomed Res. 1984;17(5):436–470. doi: 10.1016/0010-4809(84)90012-0. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum AL, Carlson MR, Gaffney R. Vertical saccadic velocity determination in superior oblique palsy. Arch Ophthalmol. 1977;95(5):821–823. doi: 10.1001/archopht.1977.04450050099011. [DOI] [PubMed] [Google Scholar]
- 6.Jampolsky A. Superior rectus revisited. Trans Am Ophthalmol Soc. 1981;79:243–256. [PMC free article] [PubMed] [Google Scholar]
- 7.Simon JW, Aaby AA, Drack AV, et al. Pediatric Ophthalmology and Strabismus. San Francisco, CA: American Academy of Ophthalmology; 2006. [Google Scholar]
- 8.Shan X, Tian J, Ying HS, et al. Acute superior oblique palsy in monkeys, I: changes in static eye alignment. Invest Ophthalmol Vis Sci. 2007;48(6):2602–2611. doi: 10.1167/iovs.06-1316. [DOI] [PubMed] [Google Scholar]
- 9.Demer JL, Miller MJ, Koo EY, Rosenbaum AL, Bateman JB. Update on Strabismus and Pediatric Ophthalmology. Boca Raton, FL: CRC Press; 1995. True vs masquerading superior oblique palsies: muscle mechanisms revealed by magnetic resonance imaging. In: Lennerstrand G, ed; pp. 303–306. [Google Scholar]
- 10.Kushner BJ. Errors in the three-step test in the diagnosis of vertical strabismus. Ophthalmology. 1989;96(1):127–132. doi: 10.1016/s0161-6420(89)32933-2. [DOI] [PubMed] [Google Scholar]
- 11.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2(1):17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 12.Kushner BJ. Ocular torsion: rotations around the “WHY” axis. J AAPOS. 2004;8 (1):1–12. doi: 10.1016/j.jaapos.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Kushner BJ. Multiple mechanisms of extraocular muscle “overaction”. Arch Ophthalmol. 2006;124(5):680–688. doi: 10.1001/archopht.124.5.680. [DOI] [PubMed] [Google Scholar]
- 14.Salmons S, Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve. 1981;4(2):94–105. doi: 10.1002/mus.880040204. [DOI] [PubMed] [Google Scholar]
- 15.Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995;36(5):906–913. [PubMed] [Google Scholar]
- 16.Demer JL, Poukens V, Ying H, Shan X, Tian J, Zee DS. Effects of intracranial trochlear neurectomy on the structure of the primate superior oblique muscle. Invest Ophthalmol Vis Sci. 2010;51(7):3485–3493. doi: 10.1167/iovs.09-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41(12):3787–3797. [PubMed] [Google Scholar]
- 18.Kono R, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior oblique muscle in superior oblique palsy. Ophthalmology. 2003;110(6):1219–1229. doi: 10.1016/S0161-6420(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 19.Rothfus WE, Curtin HD. Extraocular muscle enlargement: a CT review. Radiology. 1984;151(3):677–681. doi: 10.1148/radiology.151.3.6546996. [DOI] [PubMed] [Google Scholar]
- 20.Jiang L, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior rectus muscle in superior oblique muscle palsy. Ophthalmology. 2008;115(11):2079–2086. doi: 10.1016/j.ophtha.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott AB. Change of eye muscle sarcomeres according to eye position. J Pediatr Ophthalmol Strabismus. 1994;31(2):85–88. doi: 10.3928/0191-3913-19940301-05. [DOI] [PubMed] [Google Scholar]