In mammals and other vertebrates the pacemaker cells of the sino-atrial (SA) node are under powerful inhibitory control of parasympathetic postganglionic cardiomotor neurones. The preganglionic neurones are located in the nucleus ambiguus; some are also located in the dorsal motor nucleus of the vagus although their function in the regulation of pacemaker activity is unknown (Jones, 2001). Activation of these preganglionic cardiomotor neurones leads to activation of cholinergic postganglionic neurones in cardiac ganglia, which in turn results in inhibition of the pacemaker cells in the SA node and subsequently in a decrease of heart rate. It is universally propagated in textbook chapters and elsewhere that the synaptic transmission from parasympathetic preganglionic neurones to postganglionic neurones in the cardiac ganglia occurs with a high safety factor without modulation and that the inhibition of pacemaker cells by activation of cholinergic cardiomotor neurones is mainly generated by the opening of potassium channels via the second-messenger adenylate cyclase/cAMP pathway and by suppression of hyperpolarization-activated Na+/K+-channels. These assumptions need to be corrected in view of experimental data published previously and recently.
Impulse transmission in parasympathetic cardiac ganglia
Intracellular measurements in neurones of an in vitro preparation of parasympathetic cardiac ganglia of the guinea pig with attached atria and attached vagosympathetic trunk have shown that the neurones consist of three types (Edwards et al. 1995). (1) Principal neurones receive synaptic input from vagal preganglionic neurones with strong afterhyperpolarization following action potentials (‘synaptic afterhyperpolarizing’ (SAH) neurones). SAH neurones show subthreshold EPSPs to electrical stimulation of local nerve fibre bundles and fire tonically to intracellular injection of depolarizing current pulses. (2) Neurones which are not synaptically activated by vagal nerve stimulation but by stimulation of local nerve fibre bundles. They fire phasically to intracellular injection of depolarizing current pulses (S (synaptic) neurones). (3) Neurones which cannot be synaptically activated by vagal nerve stimulation or by stimulation of local nerve fibre bundles. They fire phasically to intracellular injection of depolarizing current pulses (P neurones since they were believed to have pacemaker properties). These three cell types show distinct morphologies. Many SAH and S cells exhibit synaptic ongoing activity in the in vitro preparation, most of it being subthreshold, whereas P cells are almost silent. Edwards et al. (1995) suggested that P neurones are afferent neurones forming synapses with SAH and S neurones and that S neurones are local cardiomotor neurones or interneurones being involved in peripheral reflex activity (Fig. 1A).
Figure 1. Integration in parasympathetic cardiac ganglia and neuroeffector transmission to cells of the sino-atrial node in guinea pig.
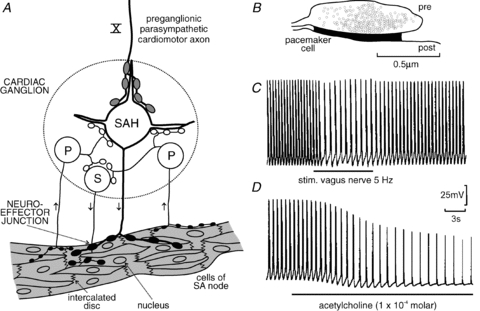
A, diagram of the component neurones of intracardiac ganglia innervating the functional syncytium of cells of the sino-atrial (SA) node: a hypothesis. Only one neurone type (SAH, synaptic with after-hyperpolarization) receives preganglionic synaptic inputs via the vagus nerve (X). Some SAH cells and all cells of another neurone type (S, synaptic) receive synaptic input arising from the third neurone type called P cells (P, pacemaker neurones). P cells may be sensory neurones with afferent terminals within the heart. The physiological stimuli to activate them are unknown. B, schematic outline of a cholinergic neuromuscular junction formed by a varicosity on a cell of the SA node based on serial sectioning and tracings of a cholinergic varicosity. These junctions cover much less than 1% of the cell surface of the pacemaker cells. Pre, prejunctional; post, postjunctional. C and D, intracellular records from a pacemaker cell in an in vitro preparation with attached vagus nerve. The cell shows pacemaker potentials with action potentials. Electrical stimulation of the vagus nerve at 5 Hz shows no change in action potential configuration and no hyperpolarization but a reduced slope of the pacemaker potential and a reduced frequency of action potentials. The mechanism is reduction of Na+ current (due to closing of voltage-dependent Na+ channels and voltage-independent background Na+ channels and increase in membrane resistance). The intracellular second-messenger pathway connecting subjunctional muscarinic ACh receptors and Na+ channels is unknown. Exogenous ACh (C) hyperpolarizes the membrane and shunts the action potentials (due to opening of K+ channels and decrease in membrane resistance), reducing their amplitude and duration. The intracellular second-messenger pathway connecting extrajunctional muscarinic ACh receptors and K+ channels is the cAMP pathway. A based on Edwards et al. (1995); B according to Klemm et al. (1992) and Choate et al. (1993); C and D modified from Campbell et al. (1989).
In this issue of The Journal of Physiology, McAllen et al. (2011) describe intracellular measurements recorded in postganglionic neurones of parasympathetic cardiac ganglia of the working heart–brainstem preparation (WHBP) of Wistar rats. About 40% of the neurones are synaptically activated by preganglionic parasympathetic cardiomotor neurones, most of them being spontaneously active. They show typical reflex activation to physiological stimulation of arterial baroreceptors, arterial chemoreceptors or trigeminal afferents. Ongoing activity and reflex activity in an individual postganglionic neurone are mediated by one preganglionic neurone. Thus, the integration of the three afferent inputs occurs at the level of the lower brainstem. The remaining 60% of the neurones in the cardiac ganglia cannot be activated by vagal preganglionic neurones and are silent. As in Edwards et al. (1995), the vagally activated neurones are tonically active to intracellular injection of depolarizing current pulses whereas the other two groups of neurone are phasically active.
Synaptic transmission from preganglionic to postganglionic cardiomotor neurones is less than 1 to 1; the EPSPs generated by activity in a single preganglionic neurone show considerable variance. This is possibly related to frequency-dependent synaptic depression, afterhyperpolarization and refractoriness of the preganglionic neurones.
What are the functions of the neurones in the parasympathetic cardiac ganglia showing no activation to stimulation of preganglionic parasympathetic neurones? Are they involved in peripheral reflex activity (Armour, 2008) and/or in modulation of synaptic activation of principal postganglionic (SAH) cardiomotor neurones? These questions have to be worked out using the WHBP and an in vitro preparation similar to that designed by Edwards et al. (1995). An interesting starting point may be the observation that many S cells in the in vitro preparation of Edwards et al. (1995) exhibit ongoing synaptic activity whereas neurones receiving no preganglionic synaptic input are silent in the WHBP (McAllen et al. 2011).
Neuroeffector transmission to pacemaker cells in the SA node
Outstanding experimental work published previously on an atrial in vitro preparation with attached vagus nerve of the guinea pig or the toad Bufo marinus conducted by the Hirst group (Bywater et al. 1989, 1990; Campbell et al. 1989; Edwards et al. 1993) shows that the mechanism underlying inhibition of pacemaker activity generated by nerve-released acetylcholine (ACh) or by ACh superfused over the preparation are fundamentally different. (1) Varicosities of postganglionic parasympathetic cardiomotor axons form synapses with the pacemaker cells covering much less than 1% of their surface (Fig. 1B; Klemm et al. 1992; Choate et al. 1993). Neuronally released ACh reacts with specialized subjunctional ACh receptors. It does not leave the synaptic cleft. This leads to inhibition of sodium channels (sodium background current, voltage-dependent sodium current) mediated by an as-yet-unknown second messenger pathway and slowing of pacemaker activity. The action potentials do not change in size and duration, the membrane does not hyperpolarize and the membrane resistance increases (due to closing of sodium channels). Neither potassium channels nor hyperpolarization-activated Na+/K+ channels seem to be involved (Fig. 1C; Bywater et al. 1989, 1990; Campbell et al. 1989; Edwards et al. 1993). (2) Exogenously applied ACh reacts with extrajunctionally located ACh receptors and causes hyperpolarization of pacemaker cells by opening of potassium channels; the membrane resistance decreases and the action potentials are reduced in size and duration (due to shunting of the membrane by opening of potassium channels). (3) In the arrested atrial preparation (action potentials prevented by blockade of calcium channels) barium ions (blocking potassium channels) prevent the hyperpolarization generated by exogenously applied ACh but do not block the effect of vagus nerve stimulation (which is now hyperpolarizing since the open sodium channels keep the membrane potential in a depolarized state of about –35 mV) (Bywater et al. 1990; Hirst et al. 1991). In conclusion neurally released ACh and exogenously applied ACh cause slowing of pacemaker cells via muscarinic ACh receptors (both effects being blocked by muscarinic antagonists) by different mechanisms, involving different cellular effectors (ionic channels) and different intracellular pathways. The mechanisms underlying the vagus-induced slowing of pacemaker activity operate in physiological conditions.
It remains to be mentioned that also hyperpolarization of pacemaker cells of the SA node to electrical stimulation of the vagus nerve has been described in young cats (Jalife & Moe, 1979). The controversial issue of the function of junctional and extrajunctional Ach receptors in pacemaker cells involving different ionic channels and intracellular pathways has been discussed by Demir et al. (1999).
Conclusions
The peripheral and central neural mechanisms of regulation of the heart in vertebrates by the autonomic nervous system are functionally highly specific (Jänig, 2006) as seen in the reflex patterns of the preganglionic (parasympathetic and sympathetic) cardiomotor neurones, the central organization of these autonomic systems, and the transmission of activity to the cardiac effector cells. Impulse transmission through cardiac ganglia is hypothesized to be modulated by mechanisms intrinsic to pre- and postganglionic cardiomotor neurones and by integrative processes involving peripheral afferent neurones, interneurones and other neurones in the ganglia (Armour, 2008). The nature of these integrative processes in the peripheral cardiac ganglia is unclear. These mechanisms have to be worked out using the WHBP and further developed in vitro preparations as shown by Hirst and co-workers. After all, about 60% of the neurones in the parasympathetic cardiac ganglia cannot be activated by stimulation of vagal preganglionic neurones. However, many of them can be synaptically activated by stimulation of intrinsic nerve fibre bundles (Fig. 1A). In which way are these neurones involved in regulation of cardiac target cells (pacemaker cells, atrial and ventricular muscle cells, blood vessels)? What are the physiological stimuli activating the intrinsic putative afferent neurones in the parasympathetic cardiac ganglia? Understanding the physiological mechanisms of impulse transmission in cardiomotor pathways will guide us to their functioning in pathophysiology.
References
- Armour JA. Exp Physiol. 2008;93:165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- Bywater RA, Campbell G, Edwards FR, Hirst GD, O'Shea JE. J Physiol. 1989;415:35–56. doi: 10.1113/jphysiol.1989.sp017710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater RA, Campbell GD, Edwards FR, Hirst GD. J Physiol. 1990;425:1–27. doi: 10.1113/jphysiol.1990.sp018089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GD, Edwards FR, Hirst GD, O'Shea JE. J Physiol. 1989;415:57–68. doi: 10.1113/jphysiol.1989.sp017711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choate JK, Klemm M, Hirst GD. J Auton Nerv Syst. 1993;44:1–15. doi: 10.1016/0165-1838(93)90374-4. [DOI] [PubMed] [Google Scholar]
- Demir SS, Clark JW, Giles WR. Am J Physiol Heart Circ Physiol. 1999;276:H2221–H2244. doi: 10.1152/ajpheart.1999.276.6.H2221. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Bramich NJ, Hirst GD. Philos Trans R Soc Lond B Biol Sci. 1993;341:149–162. doi: 10.1098/rstb.1993.0099. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Klemm MF, Steele PA. J Physiol. 1995;486:453–471. doi: 10.1113/jphysiol.1995.sp020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Edwards FR, Bramich NJ, Klemm MF. News Physiol Sci. 1991;6:185–190. [Google Scholar]
- Jalife J, Moe GK. Circ Res. 1979;45:595–608. doi: 10.1161/01.res.45.5.595. [DOI] [PubMed] [Google Scholar]
- Jänig W. The Integrative Action of the Autonomic Nervous System. Neurobiology of Homeostasis. Cambridge, New York: Cambridge University Press; 2006. [Google Scholar]
- Jones JF. Exp Physiol. 2001;86:797–801. doi: 10.1111/j.1469-445x.2001.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Klemm M, Hirst GD, Campbell G. J Auton Nerv Syst. 1992;39:139–150. doi: 10.1016/0165-1838(92)90054-k. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Salo LM, Paton JFR, Pickering AE. J Physiol. 2011;589:5801–5817. doi: 10.1113/jphysiol.2011.214320. [DOI] [PMC free article] [PubMed] [Google Scholar]


