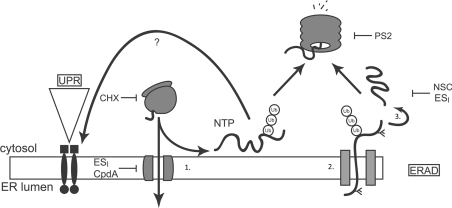
Figure 6. Proposed model for cellular actions of ESI.
Proteins possessing an ER signal sequence are targeted to the ER and translocated through the Sec61 translocon (1). Properly folded proteins exit the ER and move along the secretory pathway. Misfolded proteins are retained by the ER quality control machinery (ER-QC) (2), handed over to the retrotranslocation machinery, polyubiquitinated and retrotranslocated (2). The retrotranslocated protein is deubiquitinated and deglycosylated (3) before being degraded by the proteasome. Accumulation of misfolded proteins in the ER lumen activates UPR signalling. ESI inhibits translocation of proteins across the ER membrane, leading to the production of non-translocated proteins (NTP) at the cytosolic face of the ER membrane. These aberrant species are degraded by the UPS and also activate the UPR through an unknown mechanism. ESI may also inhibit one or more steps of the ERAD pathway, including retrotranslocation or deubiquitination. Also shown are the points of action of CHX, PS2 and NSC687852 (NSC), a deubiquitinase inhibitor that induces accumulation of polyubiquitin in cells [18].