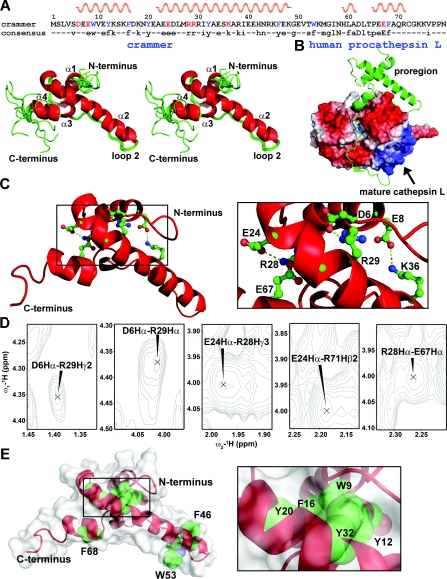
Figure 5. Solution structure of C72S.
(A) Stereo view of eleven lowest-energy structures that are superpositioned on their backbone atoms (N, Cα and C'). The helices α1, α2, α3 and α4 are labelled. The consensus sequence is shown at the top of the Figure, with the helical regions indicated. (B) Structure of human procathepsin L (PDB code 1CS8). The proregion is shown in ribbon representation. (C) The crammer residues involved in salt bridges are shown as ball-and-stick representation in the inset. (D) NOE cross-peaks for the charged side-chains that are involved in salt-bridge formation. (E) The aromatic residues in C72S are shown in spheres to indicate the hydrophobic core packing. The protein surface is shown in white.