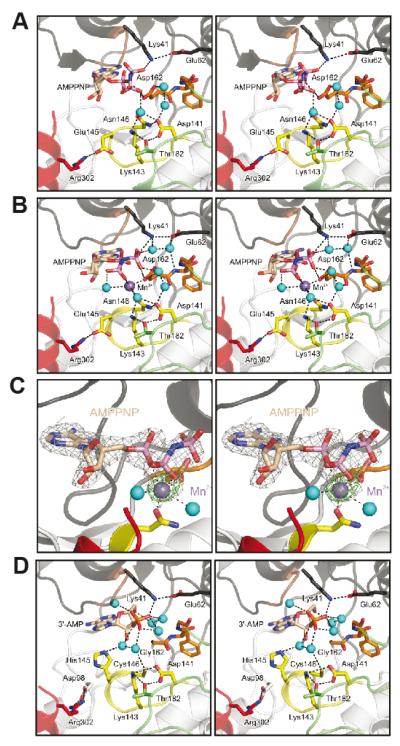
Figure 4. Nucleotide-binding pocket of the CASK4M CaM-kinase domain.
A. CASK4M CaM-kinase in complex with AMPPNP without a divalent metal ion.
B. CASK4M CaM-kinase in complex with AMPPNP-Mn2+. Residues of the Mg2+-binding loop are shown in orange, residues of the catalytic loop are in yellow and residues of the C-terminal Ca2+/CaM-binding element are in red (as in Figure 3). Selected residues and the nucleotides are shown as sticks and colored by atom type; carbon - as the respective fragment; oxygen - red; nitrogen - blue; phosphorus - pink. Water molecules (cyan) and the Mn2+ ion (purple) are shown as spheres. The orientations are the same as in Figure 3A left panel. The orientations of the AMPPNP β and γ-phosphates differ in the complexes without and with Mn2+ (compare panels A and B).
C. Stereo plot showing the final 2Fo-Fc electron density around the AMPPNP-Mn2+ complex contoured at the 1 σ level (gray mesh) and the anomalous difference Fourier map contoured at the 5 σ level (green mesh), indicating the position of the Mn2+ ion. AMPPNP is shown as sticks and colored by atom type as before. The Mn2+ ion (purple) and two coordinating water molecules (cyan) are shown as spheres. The orientation is the same as in Figure 3A left panel.
D. CASKWT CaM-kinase domain in complex with co-purified 3′-AMP (21). Color-coding as above. The orientations are the same as in Figure 3B left panel.