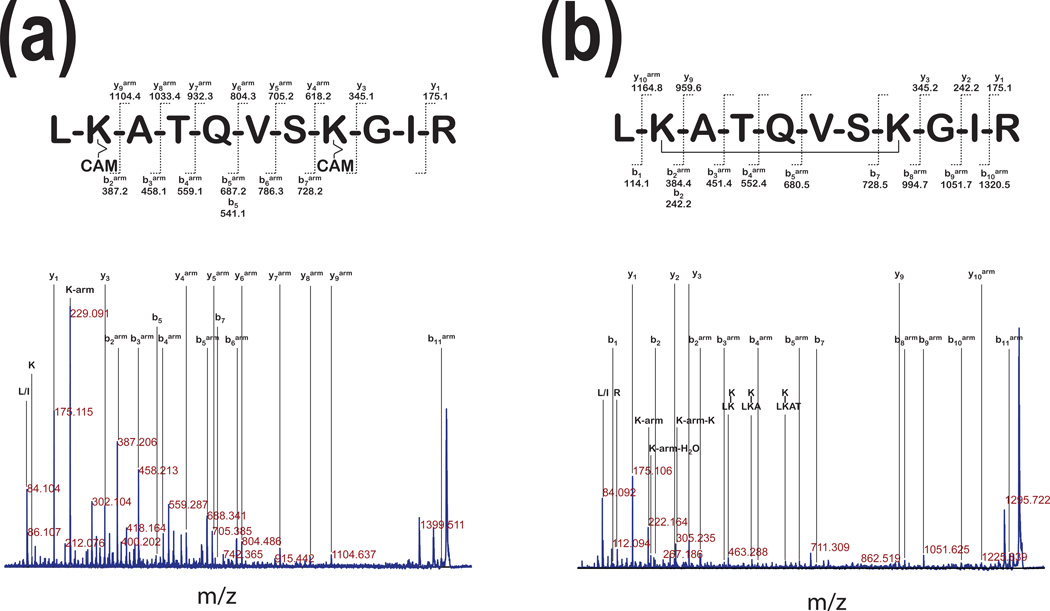
Figure 2. Comparison of cross-linking with cleavable or non-cleavable linkers.
Panel a: The MS/MS analysis of precursor ions with 1490.7 m/z (~86 kDa, Fig. 1a) of pGSN after linking with a cleavable 12Å cross-linker shows fragment ion assignment for peptideH2N-LKATQVSKGIR-COOH with modifications resulting from the spacer arm cleaved by reduction and a carbamidomethylation (CAM) of the free -SH. In this spectrum, a 229.09 m/z fragment corresponds to the immonium ion of the lysine residue linked to the arm with the CAM. Panel b: The MS/MS analysis of precursor ions with 1338.7 m/z from the digest of the major band of pGSN cross-linked with a 11.4 Å linker, shows fragment ion assignment for the same peptide as in panel (a). This peptide contains non-cleavable 11.4Å linker. This MS/MS spectrum shows ions specific for the following modifications: 222.16 m/z, the immonium ion of lysine with the arm; 242.20 m/z, the immonium ion of lysine linked to the arm and water; 305.24 m/z the arm linked on both sides to immonium ions of lysine. The lysine residue modifications were labeled with a superscript (arm).