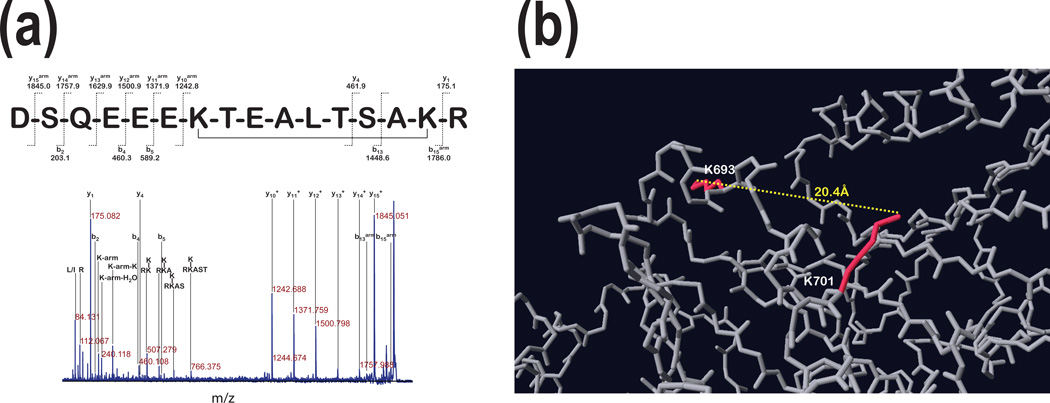
Figure 3. Internal cross-linking distant lysine residues using non-cleavable linker.
Panel a: The MS/MS analysis of the precursor ion with 1959.89 m/z (~86kDa in Fig. 1 a) linked with 11.4Å linker presents fragment ion assignment for peptide H2N-DSQEEEKTEALTSAKR-COOH with the cross-linker molecule linking both lysine residues present in this peptide. The ions containing the mass of the arm were labeled with subscript (arm). Panel b shows that the linked lysine side chains are in opposite directions and the distance between the ε-amines of both lysine residues is 20.4Å.