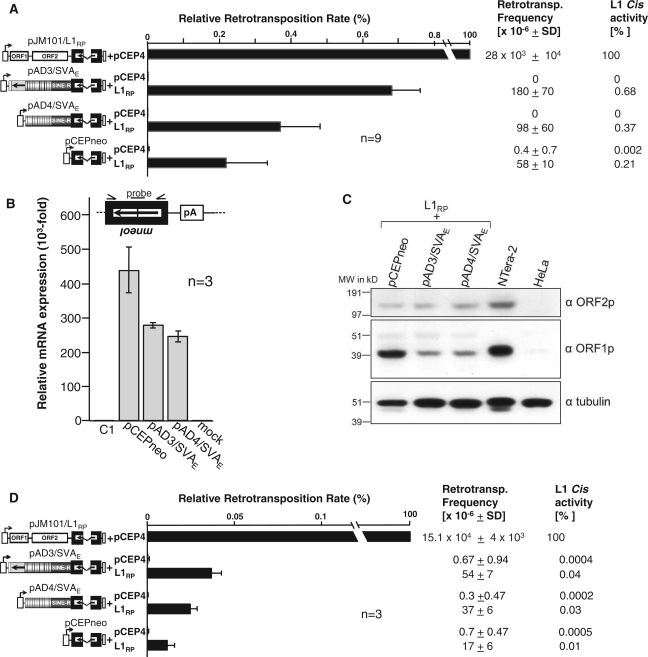
Figure 2.
Trans-mobilization of mneoI-tagged SVAE reporter elements. (A) SVAE retrotransposition reporter assay after hygromycin selection for the presence of expression plasmids. SVAE reporter plasmids pAD3/SVAE, pAD4/SVAE, or pCEPneo were cotransfected with the L1 protein donor pJM101/L1RPΔneo (L1RP) or the empty vector pCEP4. After hygR selection, G418R selection for retrotransposition events followed and retrotransposition rates were determined by counting G418R HeLa colonies. Each cotransfection experiment and subsequent retrotransposition reporter assay was carried out three times in triplicates. Retrotransposition frequencies per 106 cells are listed and relative retrotransposition rates are indicated as bar diagram. Cis retrotransposition rate of the L1 reporter element pJM101/L1RP was set as 100%. Each bar depicts the arithmetic mean ± SD of the relative retrotransposition rates obtained from nine individual cotransfection experiments (n = 9). (B) qRT–PCR analyses to quantify the relative amounts of spliced transcripts expressed from retrotransposition reporter cassettes. Total RNA was isolated 48 h after cotransfection of pCEPneo, pAD3/SVAE, and pAD4/SVAE with the L1 protein donor plasmid pJM101/L1RPΔneo. The used primer/probe combination (see ‘Materials and Methods’ section) is specific for the spliced mneoI-cassette (black box with arrow). Relative amounts of mRNA expression refer to the signal obtained from total RNA of untransfected HeLa cells which was set as 1 (C1); Total RNA from mock-transfected HeLa cells served as negative control. (C) Immunoblot analysis of L1 protein expression in HeLa cells after cotransfection of the L1 protein donor (L1RP) with retrotransposition reporter plasmids pAD3/SVAE, pAD4/SVAE and pCEPneo. Whole-cell lysates were prepared 14 days after cotransfection upon completion of hygromycin selection and subjected to immunoblot analysis using antibodies against either L1 ORF1p (αORF1p) or L1 ORF2p (αORF2p). An amount of 20 µg of whole-cell extracts were loaded per lane. α-tubulin protein levels (~50 kDa) were analyzed as loading control. Lysates from untransfected HeLa cells and from the germ cell tumor cell line NTera-2 served as negative and positive control for L1 protein detection, respectively. (D) SVAE trans-mobilization assay after transient cotransfection of expression plasmids. pAD3/SVAE, pAD4/SVAE or pCEPneo were transiently cotransfected with pJM101/L1RPΔneo (L1RP) or pCEP4 into HeLa cells. Two days later, cells were G418-selected for de novo retrotransposition events for 14 days. G418R HeLa colonies were Giemsa-stained and counted. Each cotransfection experiment was done in quadruplicate. Subsequent retrotransposition reporter assays were performed in triplicate. Retrotransposition frequencies per 106 cells are listed and relative retrotransposition rates are indicated as bar diagram. Cis retrotransposition rate of L1 reporter element pJM101/L1RP was set as 100%. Each bar depicts the arithmetic mean ± SD of the relative retrotransposition rates obtained from three individual cotransfection experiments (n = 3).