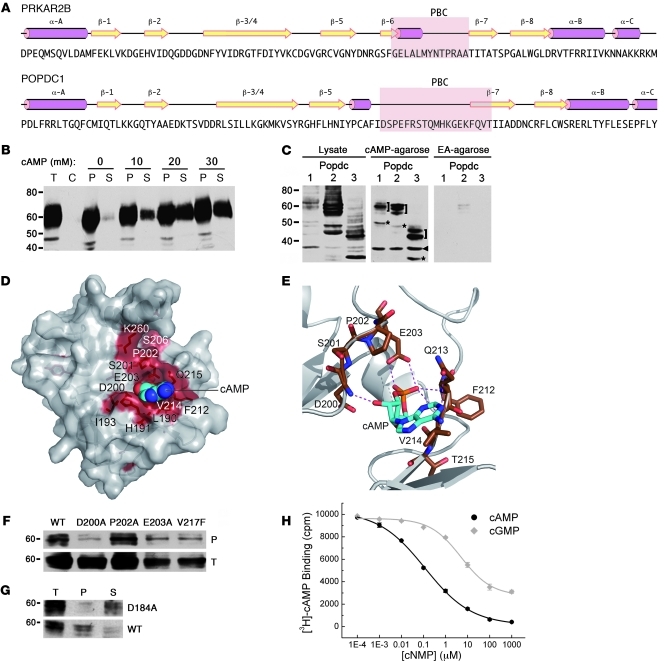
Figure 4. The Popeye domain functions as a cyclic nucleotide-binding domain.
(A) Secondary structure of human cAMP-dependent protein kinase type II-β regulatory subunit (PRKAR2B) and structural prediction of the Popeye domain of human POPDC1. Pink rods, α helices; yellow arrows, β strands; pink underlay, PBC. (B) Western blot detection of chick Popdc1 after affinity precipitation of cardiac tissue extracts incubated with cAMP-agarose. Bound Popdc1 protein was eluted with increasing amounts of cAMP. P, pellet; S, supernatant; T, total protein; C, control incubation using ethanolamine-agarose. (C) Western blot of Cos-7 cells transfected with Popdc1, Popdc2, and Popdc3 cDNAs. Shown are total protein and protein bound to cAMP-agarose or ethanolamine (EA) agarose. Brackets denote differentially glycosylated Popdc proteins; asterisks denote nonglycosylated form of Popdc proteins; arrowhead denotes unspecific immunoreactive protein. (D) 3D model of the Popeye domain of human POPDC1. Invariant amino acids are colored red. The cAMP moiety is shown as CPK model. (E) Enlargement of the predicted cAMP binding site. Predicted hydrogen bonds between cAMP and the DSPE and FQVT sequence motifs are shown as dashed lines. (F) Western blot of cAMP-agarose precipitations of Cos-7 cells transfected with cDNAs encoding Popdc1 and Popdc1D200A, Popdc1P202A, Popdc1E203A, or Popdc1V217F mutants. (G) Western blot of cAMP-agarose precipitations of Cos-7 cells transfected with cDNAs encoding Popdc2 or Popdc2D184A mutant. (H) Radioligand binding assay using [3H]-cAMP and recombinant C terminus of Popdc1. Binding was competed with increasing concentrations of free unlabeled cAMP and cGMP, respectively.