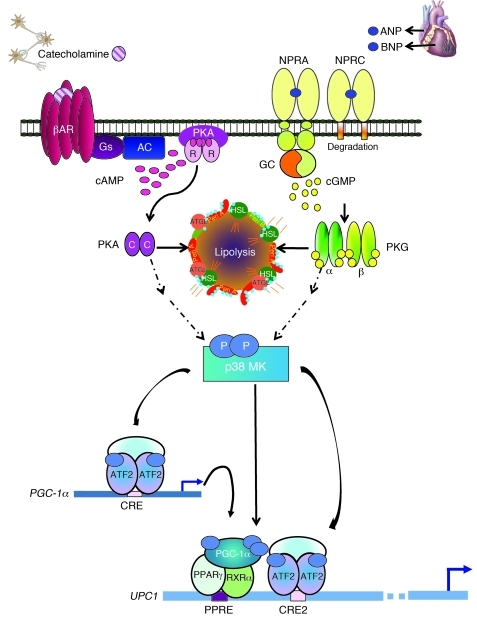
Figure 12. Model for parallel β-AR and NPRA activation of p38 MAPK to trigger expression of the brown fat thermogenic gene program.
Catecholamines bind the heptahelical β-ARs on adipocytes to activate the G protein Gs and increase cAMP (pink ovals). cAMP binds the regulatory (R) subunits of PKA. The released catalytic (C) subunits (purple ovals) can then phosphorylate targets, including HSL and perilipin (Peri A), to allow lipolysis of stored triglycerides. Lipolysis can also be activated by NPs. ANP and BNP bind to guanylyl cyclase (GC) receptor NPRA to increase cGMP (yellow circles). Adipocytes also express NPRC that mainly removes NPs from circulation. The cGMP produced by NPRA activates PKG (α and β subunits are represented as thin green and yellow ovals, respectively), whose substrate specificity for phosphorylation closely overlaps that of PKA. Thus, PKG can phosphorylate the same targets as PKA to elicit lipolysis. β-ARs and PKA can also activate a protein kinase cascade, culminating in the activation of p38α MAPK (p38 MK). Now, we can add NPs and PKG as parallel activators of p38α MAPK. Thus, in response to β-agonist or NPs, p38α MAPK phosphorylates (light blue ovals) the transcriptional regulators ATF2 and PGC-1α. PGC-1α interacts with PPARγ and RXRα. These phosphorylated and activated factors are recruited to specific motifs within the UCP1 enhancer (PPRE, CRE2) to increase its gene expression (blue arrow). ATF2 also binds the CRE in the PGC-1α promoter to increase transcription (blue arrow) and increase the amount of PGC-1α.