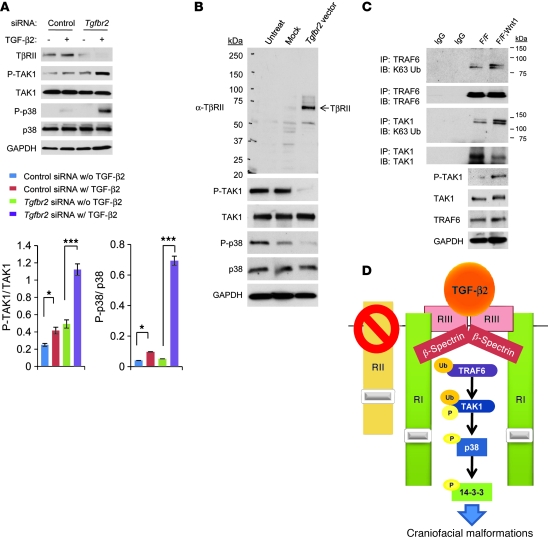
Figure 4. TβRII is crucial in regulating TAK1/p38 MAPK activity.
(A) Immunoblotting analysis of indicated molecules in primary MEPM cells from Tgfbr2fl/fl control mice treated with control or Tgfbr2 siRNA and cultured with (+; w/) or without (–; w/o) TGF-β2 (10 ng/ml) for 30 minutes. The bar graphs show the ratios of phosphorylated TAK1 relative to TAK1 and phosphorylated p38 relative to p38 after quantitative densitometry analysis of immunoblotting data. Three samples were analyzed for each experiment. Error bars represent SD. *P < 0.05; ***P < 0.001. (B) Immunoblotting analysis with indicated antibodies of untreated Tgfbr2fl/fl;Wnt1-Cre MEPM cells (Untreat) or empty vector treated Tgfbr2fl/fl;Wnt1-Cre MEPM cells (Mock) or cells after reintroduction of Tgfbr2 (Tgfbr2 vector). (C) Immunoblotting analysis with anti-K63 ubiquitin (K63 Ub) antibody after immunoprecipitation by anti-TRAF6 or anti-TAK1 antibody of extracts from MEPM cells from Tgfbr2fl/fl (F/F) and Tgfbr2fl/fl;Wnt1-Cre (F/F;Wnt1) mice. (D) Schematic diagram depicts our model of the mechanism of p38 MAPK activation in the Tgfbr2fl/fl;Wnt1-Cre palate, leading to craniofacial malformations. TGF-β2 is upregulated and binds to TβRIII (RIII), followed by assembly with TβRI (RI) and β-spectrin. TRAF6 activates TAK1 ubiquitination (Ub) and phosphorylation (P) after TGF-β2 binding. Finally, p38 and 14-3-3 proteins are phosphorylated, leading to downstream signaling and cell proliferation defect. RII, TβRII.