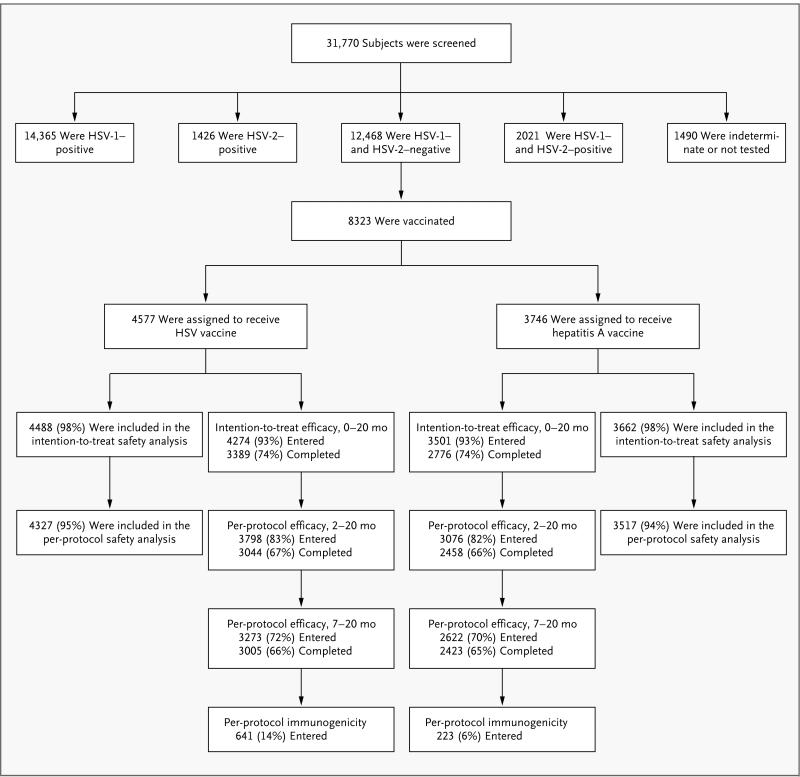
Figure 1. Randomization of Study Subjects.
Both modified intention-to-treat and per-protocol analyses of efficacy were performed. Before the treatment assignments were revealed, an audit showed serious protocol violations at a single site; the sponsors declared that data from subjects at this site could not be evaluated for efficacy. The intention-to-treat analysis included all vaccinated subjects except those at the site with serious protocol violations, those who were found to be HSV-seropositive at study entry, and those who did not return to the clinic after vaccination. The per-protocol analyses were further restricted to subjects who received either two doses of vaccine (per-protocol cohort for months 2 through 20) or three doses (per-protocol cohort for months 7 through 20) within protocol-specified windows, were uninfected at the start of each respective risk period, and did not have prespecified protocol deviations that might have confounded assessment of exposure, immunogenicity, or efficacy. Immunogenicity was evaluated in a random sample of subjects in the per-protocol cohort for months 7 through 20, with censoring of results after infection. A total of 31,770 women were screened by Western blot analysis for herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) antibodies for the Herpevac Trial, of whom 39% were seronegative for both and 26% were vaccinated in the trial. The target treatment-assignment ratio was 1:1, but owing to a programming error, the initial subjects were randomly assigned at a 3:1 ratio in favor of the HSV-vaccine group. The data safety and monitoring board identified the problem, and randomization was corrected to a 1:1 ratio for the balance of the trial, resulting in a final ratio of 55:45 in favor of the HSV-vaccine group.