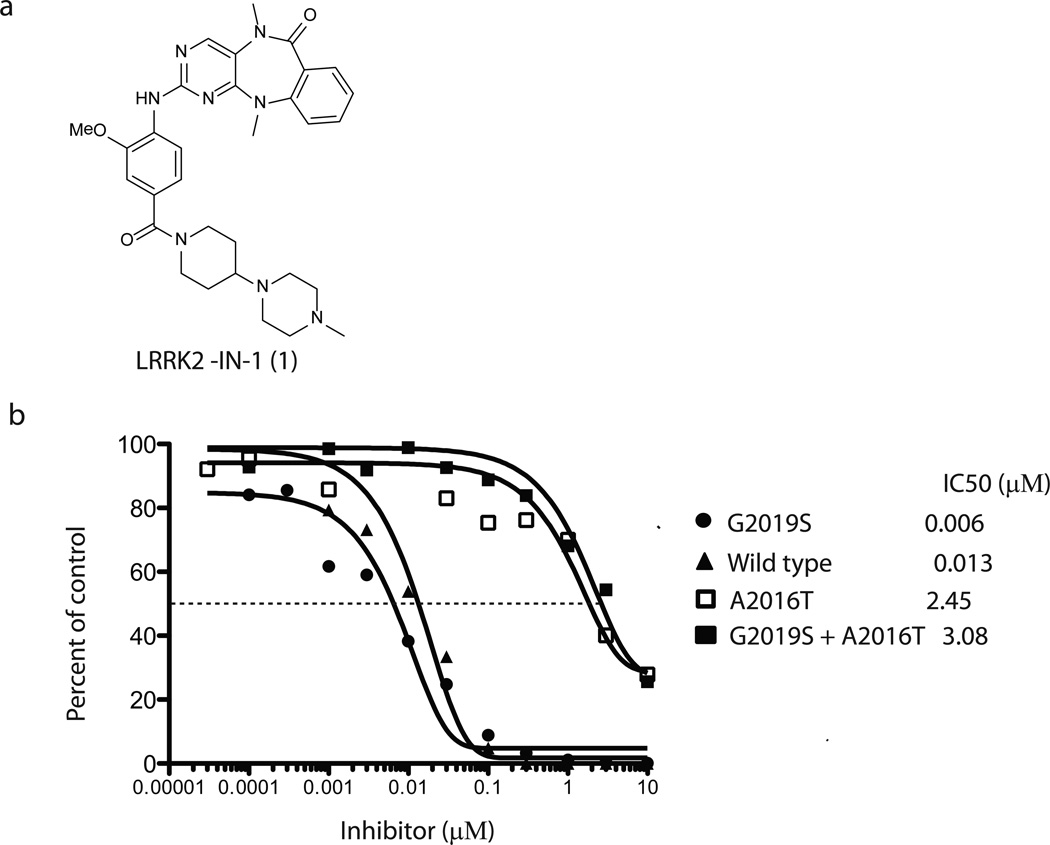
Figure 1. Enzymatic activity of LRRK2-IN-1 and its selectivity.
(a) Chemical structure of the LRRK2 inhibitor LRRK2-IN-1. (b) GST-LRRK2 (1326-2517), GST-LRRK2 [G2019S] (1326-2527), GST-LRRK2 [A2016T] (1326-2517) and GST-LRRK2 [A2016T+G2019S] (1326-2517) were assayed using 20µM Nictide in the presence of 100 µM ATP with the indicated concentrations of LRRK2-IN-1. The results are presented as percentage of kinase activity relative to the DMSO treated control. Results are averages of duplicate reactions with similar results obtained in at least one other experiment. The IC50 values, in µM, were derived from the graphs. (c) The combined kinase profiling hits with biochemical activities. Kinase hits from Ambit profiling with a score of less than 10% of the DMSO control at a concentration of 10 µM, ActivX profiling with higher than 50% inhibition at 1 µM, and Dundee profiling with greater than 50% inhibition at 1 µM are listed. The biochemical IC50 values of hits were measured by Invitrogen Adapta®, Z´-Lyte®, Lantha-Screen® assays (http://www.invitrogen.com/site/us/en/home/Products-and-Services/Services/Screening-and-Profiling-Services/SelectScreen-Profiling-ervice/SelectScreen-Kinase-Profiling-Service.html) and Dundee radioactive-base enzyme assay14. *Assessment of PLK1 cellular activity of LRRK2-IN-1 (see Supplementary Results).