SUMMARY
Long-term storage of episodic memories is hypothesized to result from the off-line transfer of information from the hippocampus to neocortex, allowing a hippocampal-independent cortical representation to emerge. However, off-line hippocampal-cortical interactions have not been demonstrated to be linked with long-term memory. Here, using functional magnetic resonance imaging, we examined if hippocampal-cortical BOLD correlations during rest following an associative encoding task are related to later associative memory performance. Our data show enhanced functional connectivity between the hippocampus and a portion of the lateral occipital complex (LO) during rest following a task with high subsequent memory compared to pre-task baseline resting connectivity. This effect is not seen during rest following a task with poor subsequent memory. Furthermore, the magnitude of hippocampal-LO correlations during post-task rest predicts individual differences in later associative memory. These results demonstrate, for the first time, the importance of post-experience resting brain correlations for memory for recent experiences.
INTRODUCTION
It has been suggested that humans have the capacity to remember daily episodes for years and even decades. This amazing feat is thought to depend upon a temporally evolving process that involves interactions between the hippocampus and neocortex. While the hippocampus is critical for the initial creation of an episodic memory trace, it is hypothesized that long-term storage results from the restructuring of information across hippocampal-neocortical networks over time, resulting in a distributed memory representation (Nadel et al., 2000; Squire et al., 1984). This restructuring has been termed memory consolidation and is thought to be mediated by ‘replay’, or the off-line reactivation of the same patterns of activity that are present during a prior experience (Marr, 1971; McClelland et al., 1995; Rasch and Born, 2007; Sutherland and McNaughton, 2000). In order to restructure information across hippocampal-neocortical networks, it is thought that reactivation is coordinated across the hippocampus and neocortex through hippocampal-cortical interactions, as well as interactions between relevant neocortical areas.
Numerous findings from rodents have provided evidence for replay during sleep. Specifically, patterns of neuronal activity that characterize waking behaviors are reactivated during subsequent sleep periods in the hippocampus (Lee and Wilson, 2002; Nadasdy et al., 1999; Pavlides and Winson, 1989; Wilson and McNaughton, 1994) and the cortex (Euston et al., 2007; Ji and Wilson, 2007; Peyrache et al., 2009; Qin et al., 1997). Additionally, reactivation has been shown to occur in a coordinated fashion between hippocampal and neocortical networks (Ji and Wilson, 2007; Qin et al., 1997) providing a potential mechanism for information transfer between hippocampal and neocortical networks. Initial support for a relationship between off-line reactivation and behavioral learning comes from a recent study in rodents (Peyrache et al., 2009) demonstrating preferential reactivation for experiences during which successful learning occurred compared to experiences without explicit learning. Furthermore, work in humans and rodents has shown a relationship between hippocampal reactivation during slow-wave sleep and subsequent memory performance (Girardeau et al., 2009; Peigneux et al., 2004; Rasch et al., 2007). Taken together, these data suggest that experience-dependent hippocampal and neocortical reactivation during sleep supports long-term memory consolidation.
It is possible that sleep is the only time when the day's experiences are strengthened in memory. Another possibility, however, is that off-line reactivation also occurs during waking periods of rest allowing for some consolidation of recent experience to occur while we are awake, as suggested by theories of memory consolidation (McClelland et al., 1995). Supporting this latter notion, reactivation of experience-dependent patterns of activity has recently been described in the rodent hippocampus and primate cortex during the awake state when animals are resting (Diba and Buzsaki, 2007; Foster and Wilson, 2006; Hoffman and McNaughton, 2002; Karlsson and Frank, 2009). Additionally, hippocampal and cortical activity has been shown to reflect patterns of activity induced by a recent task while humans are awake and performing and unrelated task (Peigneux et al., 2006). In contrast to sleep, however, reactivation during rest has not been shown to have any behavioral consequences for memory. Similarly, robust BOLD correlations between the hippocampus and cortex and between cortical areas during rest has been observed in humans (Vincent et al., 2006) but the behavioral role of these correlations is unclear (Buckner and Carroll, 2007). Two recent studies have demonstrated that resting state functional connectivity in the so-called default network can be modified by recent experience (Albert et al., 2009; Hasson et al., 2009). However, it remains unknown whether functional connectivity between hippocampal and cortical brain areas during rest are related to long-term memory consolidation.
In the present study, we examined whether resting BOLD correlations, or functional connectivity, between hippocampal-cortical and cortico-cortical regions during post-experience rest relate to later memory for those experiences. We reasoned that if inter-regional interactions during rest are important for memory consolidation, functional connectivity should be enhanced during rest following a task with high subsequent memory, compared to the baseline levels of functional connectivity during rest before the task. Furthermore, experiences followed by rest periods with higher hippocampal-cortical and cortico-cortical correlations should be better remembered than those experiences followed by lower levels of correlated activity. To this end, the first goal of the study was to determine if post-experience resting functional connectivity is elevated following a task with high levels of later associative memory, as compared to the level of correlation between these areas during a pre-task baseline rest and during post-experience rest following a task with lower levels of later associative memory. Second, we hypothesized that differences in the magnitude of post-task functional connectivity should be predictive of later individual differences in associative memory for prior task elements.
RESULTS
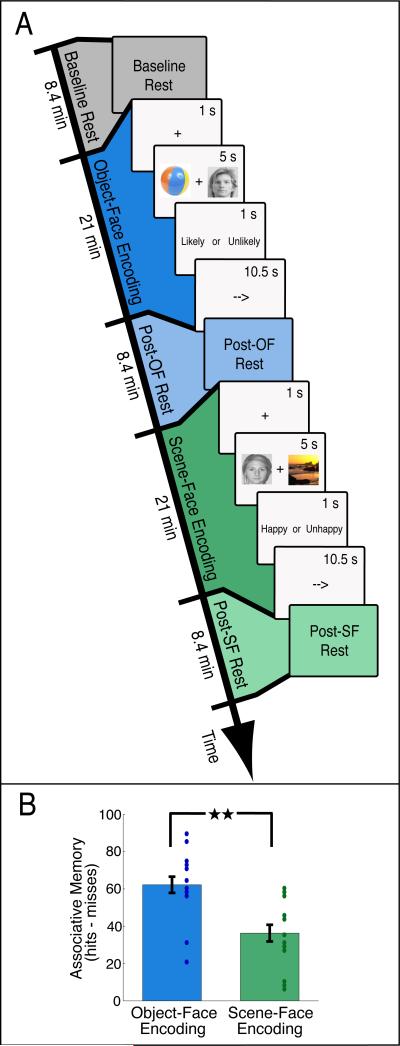
To test these predictions, we scanned sixteen human subjects using functional magnetic resonance imaging (fMRI) during the performance of two different associative encoding tasks and during pre-task and post-task rest periods (Figure 1A). Subjects were first scanned during a ‘Baseline Rest’ period to determine baseline levels of functional connectivity between hippocampal and cortical regions of interest (ROIs). Subjects then performed an object-face processing task (‘Object-Face Encoding’), immediately followed by another rest scan (‘Post-OF Rest’), a scene-face processing task (‘Scene-Face Encoding’), and another rest period (‘Post-SF Rest’). Both tasks required subjects to form an association between each object-face or scene-face pair (see Experimental Procedures). The order of the tasks was counterbalanced across subjects. In order to isolate cortical areas differentially involved in processing task stimuli, we independently localized the fusiform face area (FFA) important for face processing (Kanwisher et al., 1997), the posterior portion of the lateral occipital complex (LO) for object processing (Grill-Spector et al., 2001; Malach et al., 1995), and the parahippocampal place area (PPA) for scene processing (Epstein and Kanwisher, 1998). These cortical ROIs were localized in separate functional scans after the encoding and rest runs using novel stimuli. After the scanning session, subjects’ associative memory for the stimulus pairs from both Object-Face and Scene-Face Encoding was tested.
Figure 1. Schematic overview of experimental tasks and behavioral results.
(A) Subjects performed Object-Face and Scene-Face Encoding tasks, interleaved with rest scans (see Experimental Procedures). Note that the order of the Encoding tasks was counterbalanced across subjects. Each trial in the Encoding tasks consisted of a fixation cue, the presentation of an object-face or scene-face pair, a ‘Likely or Unlikely ’ or ‘Happy or Unhappy’ judgment, and then a baseline “arrows” task. (B) Behavioral performance on a subsequent memory test for stimuli presented during Object-Face and Scene-Face Encoding. A significant difference in associative memory (see Experimental Procedures) was found between stimuli in the Encoding tasks (★★P < 10-5). All error bars indicate mean ± standard error of the mean. Dots show data from individual subjects.
In order to investigate whether changes in functional connectivity are related to subsequent memory for task elements, we asked if associative memory performance differed between the Object-Face and Scene-Face Encoding tasks. Notably, significantly better associative memory was found for object-face pairs relative to scene-face pairs (t15 = 6.73, P < 10-5) (Figure 1B). Differences in associative d’ (calculated from the proportion of associative hits and associative false alarms) were also observed between object-face pairs (d’ = 1.48 ± .14) and scene-face pairs (d’ = 0.48 ± .08). This difference in associative memory across the two tasks allowed us to investigate the extent to which correlations between ROIs during rest are related to later memory for task elements.
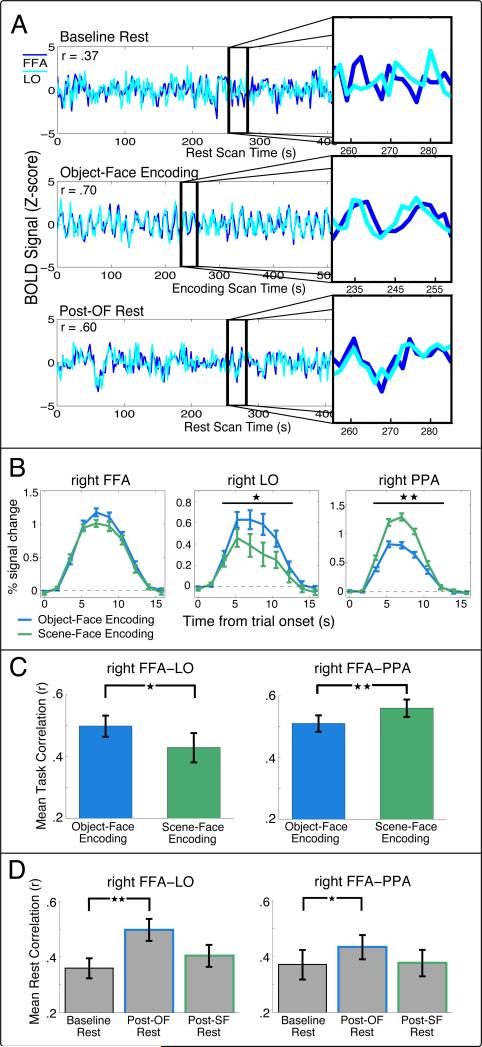
To address our first goal, we asked if resting functional connectivity between ROIs is enhanced following experiences with high levels of later associative memory (Object-Face Encoding) compared to baseline resting connectivity. We first focused on cortical regions differentially associated with the processing of task stimuli (FFA, LO, and PPA). To measure functional connectivity between cortical ROIs we computed pair-wise correlations between the time course of the blood oxygen level-dependent (BOLD) signal between the FFA and LO (FFA-LO) and the FFA and PPA (FFA-PPA) during each of the encoding tasks and rest scans, as shown for an example subject in Figure 2A. We first verified that all cortical ROIs (FFA, LO, PPA) and ROI pairs (FFA-LO and FFA-PPA) were significantly active (above fixation baseline; Figure 2B) and inter-correlated (Figure 2C) during both encoding tasks. Critically, we then examined functional connectivity during the rest period following the Object-Face Encoding (Post-OF Rest) since associative memory for object-face stimulus pairs was significantly greater than scene-face stimulus pairs. We found that functional connectivity between cortical regions was significantly enhanced in the Post-OF Rest period compared to the Baseline Rest period (before the task) (FFA-LO, t13 = 3.61, P < .005; FFA-PPA, t15 = 2.67, P < .05; Figure 2D). Note that all statistics were performed on Fisher Z-transformed correlation values (see Experimental Procedures). These significant differences were confirmed via non-parametric randomization tests (Figure S2, see Experimental Procedures).
Figure 2. Cortical activity and correlations during Encoding tasks and Rest.
(A) Example time courses and analysis procedure for an ROI pair from a single subject. The BOLD time course is shown for the FFA and LO during Baseline Rest (top), Object-Face Encoding (middle), and Post-OF Rest (bottom). The insets show time courses from the two ROIs for 29.75 s and illustrate the pattern of non-zero correlations at baseline rest, task-evoked correlations during encoding, and enhanced correlations during rest following encoding. (B) Mean BOLD responses during Encoding tasks for localizer defined ROIs. The right FFA (left), LO (middle), and PPA (right) were significantly active above baseline during both Object-Face Encoding (FFA, t15 = 14.24, P < 10-9; LO, t13 = 5.25, P < .001; PPA, t15 = 11.85, P < 10-8) and Scene-Face Encoding tasks (FFA, t15 = 13.75, P < 10-9; LO, t13 = 2.94, P < .02; PPA, t15 = 15.98, P < 10-10). No significant difference in percent signal change was found between the Encoding tasks in the right FFA (t15 = 1.57, P > .13). The right LO was significantly more active during Object-Face versus Scene-Face Encoding (t13 = 3.17, ★P < .008), with the opposite pattern found for the right PPA (t15 = 6.45, ★★P < 10-4). Significance was evaluated by comparing the area under the curve for each Encoding task across subjects. (C) Mean correlations during Object-Face and Scene-Face Encoding tasks for the right FFA-LO (left) and the right FFA-PPA (right). Higher correlations between the FFA and LO were found for Object-Face versus Scene-Face Encoding (t13 = 2.37, ★P < .05) and higher correlations between the FFA and PPA were present for Scene-Face relative to Object-Face Encoding (t15 = 3.59, ★★P < .005). Note that all statistics were computed on Fisher Z-transformed correlation (r) values. (D) Mean correlations between the right FFA-LO (left) and right FFA-PPA (right) during baseline and post-task rest periods. Greater correlations were found across subjects for the Post-OF Rest compared to the Baseline Rest for both ROI pairs. No changes were found between the Baseline Rest and Post-SF Rest.
Next, we tested whether the enhanced cortico-cortical connectivity seen in the preceding analysis is found following any task that engages these cortical regions, irrespective of later memory for task elements. We thus examined resting functional connectivity for ROI pairs after Scene-Face Encoding (Post-SF Rest), as all cortical ROIs were engaged during Scene-Face Encoding, but significantly lower memory was found for scene-face relative to object-face pairs (Figure 1B). In contrast to the results seen during Post-OF rest, we did not see evidence for enhanced connectivity during Post-SF Rest compared to Baseline Rest for any of the cortical ROI pairs examined using either parametric (Figure 2D; right FFA-LO, t13 = 1.23, P > .23; right FFA-PPA, t15 = .14, P >.88) or non-parametric tests (Figure S2, see Experimental Procedures). It is important to note that the lack of enhanced connectivity following Scene-Face Encoding was not due to a lack of engagement of the FFA, LO, or PPA (Figure 2B) or a lack of correlated activity between ROI pairs during task performance (Figure 2C). In fact, as expected, the PPA was significantly more active (Figure 2B, right panel) and significantly more correlated with the FFA (Figure 2C, right panel) during Scene-Face Encoding than during Object-Face Encoding. These results suggest that differential patterns of connectivity between ROI pairs can be found between encoding and post-task rest periods, with enhanced FFA-PPA connectivity selectively occurring during rest following Object-Face Encoding, despite greater FFA-PPA connectivity during Scene-Face versus Object-Face Encoding.
We then performed several ANOVAs in order to verify that distinct patterns of connectivity were seen during encoding and post-task rest, in particular for FFA-PPA correlations. In order to investigate differential patterns of connectivity for Object-Face and Scene-Face tasks across encoding and rest periods, we first performed a three-way repeated measures ANOVA on the correlations between ROI pairs with factors of Time Period (Encoding/Rest), Task (Object-Face/Scene-Face), and ROI pair (FFA-LO/FFA-PPA). This analysis revealed a significant interaction of Time Period, Task, and ROI pair (F1,13 = 5.64, P < .05), indicating that differential task-related connectivity was evident across ROI pairs and encoding and rest periods. To confirm that this interaction was being driven by differential patterns of connectivity specifically for the FFA-PPA ROI pair, we performed a two-way ANOVA on the FFA-PPA connectivity with factors of Time Period and Task. As expected, this ANOVA showed a significant interaction between Time Period and Task (F1,16 = 6.86, P < .02), reflecting differential patterns of FFA-PPA connectivity during the Object-Face and Scene-Face tasks across encoding and rest periods. No interaction was found between Time Period and Task for the FFA-LO connectivity (F1,13 = .16, P > .6), with a main effect of Task (F1,13 = 7.31, P < .02) indicating higher FFA-LO connectivity during both Object-Face Encoding and Post-OF Rest periods compared to Scene-Face Encoding and Post-SF Rest. Thus, these findings suggest that the pattern of functional connectivity during post-task rest does not always mirror the pattern present during the immediately preceding encoding task, but instead may be related to levels of later memory for preceding experiences.
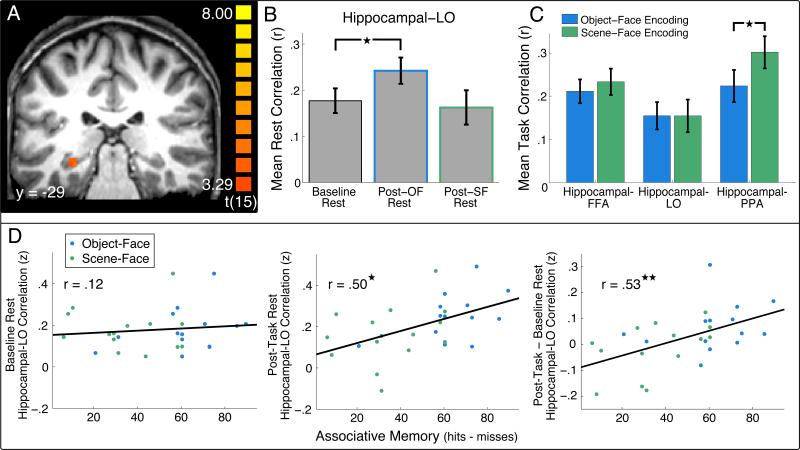
Of critical relevance to theories of memory consolidation, we next asked if enhanced hippocampal-cortical interactions are evident generally during post-experience rest and, if so, whether these interactions are related to future memory for previous experiences. To investigate hippocampal-cortical functional connectivity during rest, a hippocampal ROI was defined based on the logic that a hippocampal region involved in the successful associative encoding of presented stimulus pairs would be the most likely region of the hippocampus to show post-task resting connectivity related to the strengthening of those associations. Thus, we identified a hippocampal ROI that showed a successful subsequent associative memory effect across both encoding tasks (Figure 3A, see Experimental Procedures). Consistent with the results for the cortical ROIs, we found significantly greater correlations between the hippocampal ROI and the right LO in the Post-OF Rest compared to the Baseline Rest (Figure 3B; t13 = 2.65, P < .02), but not for the Post-SF Rest compared to the Baseline Rest (Figure 3B; t13 = .57, P > .57). We then verified that correlations between the hippocampal ROI and the right LO were significantly greater during Post-OF Rest compared to Post-SF Rest (t13 = 3.39, P < .005). Interestingly, no enhanced correlations from the Baseline Rest to the Post-OF or Post-SF Rest were found for the hippocampal ROI with the other cortical ROIs examined (FFA, PPA; Table 1) suggesting that LO may be particularly important in supporting long-term memory of stimulus pairs used in our task.
Figure 3. Hippocampal-cortical correlations during Rest and Encoding tasks.
(A) Hippocampal ROI identified from a subsequent associative memory contrast of associative hits > associative misses shown in coronal section on a high resolution anatomical image from an individual subject. Color indicates the value of the t-statistic from the contrast of associative hits – associative misses. (B) Mean BOLD correlation across subjects between the hippocampal ROI and LO during baseline and post-task rest periods. A significant increase in correlation was found from the Baseline Rest to the Post-OF Rest (★P < .05). All comparisons between correlation values were performed on Fisher Z-transformed correlation (r) values. (C) Mean correlations during Object-Face and Scene-Face Encoding tasks between the hippocampal ROI and the right FFA (left), LO (middle), and PPA (right). Significantly higher hippocampal-PPA correlations were found during Scene-Face compared to Object-Face Encoding (★P < .05). (D) Correlation between associative memory performance and hippocampal-LO BOLD resting correlations across individual subjects. During Baseline Rest, no significant correlation was seen between hippocampal-LO correlations and associative memory (left). For the post-task rest (Post-OF and Post-SF Rest), a significant correlation was found with associative memory for object-face and scene-face pairs (★P < .05, middle). The difference in hippocampal-LO correlations between post-task (Post-OF and Post-SF Rest) and Baseline Rest was also significantly correlated with associative memory (★★P < .005, right). All correlation values are Z-transformed correlation (r) values. Blue data points indicate Object-Face associative memory and Post-OF Rest correlations while green data points indicate Scene-Face memory and Post-SF Rest correlations.
Table 1.
Differences between Baseline Rest and Post-Task Rest ROI pair correlations.
| ROI Pair | Post-OF Rest vs. Baseline Rest | Post-SF Rest vs. Baseline Rest |
|---|---|---|
| Hippocampal ROI and right FFA | t15 = 1.16, P > .26 | t15 = .83, P > .42 |
| Hippocampal ROI and right PPA | t15 = 1.41, P > .17 | t15 = 1.25, P > .23 |
| PFC ROI and right FFA | t15 = 2.26, P < .04* | t15 = .38, P > .89 |
| PFC ROI and right LO | t13 = 3.49, P < .004** | t13 = 1.91, P > .078 |
| PFC ROI and right PPA | t15 = 2.75, P < .02* | t15 = 1.37, P > .18 |
We then asked if differential hippocampal-cortical correlations were found across Object-Face and Scene-Face Encoding, similar to the differential patterns of cortico-cortical correlations during the encoding tasks. As is shown in Figure 3C, no significant differences in hippocampal-FFA and hippocampal-LO correlations were found between Object-Face and Scene-Face Encoding (hippocampal-FFA, t15 = 1.05, P > .3; hippocampal-LO, t13 = .08, P > .9). However, significantly higher correlations were found between the hippocampal ROI and the PPA during Scene-Face versus Object-Face Encoding (t15 = 3.22, P < .006). The finding of similar correlations between the hippocampal ROI and LO for both Object-Face and Scene-Face Encoding suggests that the enhanced hippocampal-LO correlations seen during Post-OF, but not during Post-SF Rest, were not simply a direct consequence of differential correlations present during the encoding tasks.
Thus, the results reported thus far support the first hypothesis that functional connectivity between cortico-cortical and hippocampal-cortical ROI pairs during post-task rest are broadly related to future memory for task elements, as indicated by enhanced correlations during rest following a task with later high associative memory (Object-Face Encoding) but no change in correlations during rest after a task with relatively poor later associative memory (Scene-Face Encoding).
Next, in order to test our second hypothesis, we investigated whether the magnitude of post-experience hippocampal-cortical resting functional connectivity predicts individual differences in later memory performance. Indeed, we found that the magnitude of Post-OF and Post-SF hippocampal-LO resting correlations predicted individual differences in later associative memory for the stimulus pairs encountered in those tasks (r = .5, t26 = 2.95, P < .007; Figure 3D, middle panel). Importantly, hippocampal-LO correlations during Baseline Rest did not predict subjects’ later associative memory performance (r = .12, t26 = .61, P > .5; Figure 3D, left panel) for these same stimulus pairs. Furthermore, the difference in magnitude between post-task and Baseline hippocampal-LO resting connectivity also predicted later associative memory (r = .53, t26 = 3.19, P < .004; Figure 3D, right panel). To ensure that this effect was not simply a by-product of the overall higher hippocampal-LO connectivity during the Post-OF Rest (Figure 3B) and better memory for object-face pairs (Figure 1B), we assessed whether hippocampal-LO correlations predicted memory for the scene-face pairs alone. Indeed, a trend for significance was found between the difference in the hippocampal-LO correlation from Baseline to Post-SF Rest and associative memory for scene-face pairs (r = .512, t12 = 2.06, P < .062). Furthermore, a similar level of correlation was found for the object-face data alone in the Post-OF rest period (correlation between object-face associative memory and Post-OF Rest hippocampal-LO correlations, r = .52, t12 = 2.11, P < .057). These results support our second hypothesis that the magnitude of post-task functional connectivity predicts individual differences in long-term memory, providing evidence that enhanced hippocampal-cortical coordination during post-task rest is related to enhanced long-term memory.
In order to examine the selectivity of hippocampal-cortical connectivity in predicting memory performance, we asked if other regions isolated from the same subsequent associative memory contrast as the hippocampal ROI would also exhibit resting correlations with cortical ROIs that predict future associative memory. A region of left lateral prefrontal cortex (PFC) was isolated from the same subsequent memory contrast as the hippocampal ROI [note: no other regions emerged from this contrast with a threshold of P < .001 (uncorrected, with a minimum of six contiguous voxels)]. While we found that overall correlations between the PFC and cortical ROIs were enhanced during the Post-OF versus the Baseline Rest (Table 1), the magnitude of the PFC-LO correlations did not predict later individual differences in associative memory (post-task minus Baseline PFC-LO correlation with associative memory, r = .05, t26 = .24, P < .8). Furthermore, the correlation between associative memory and hippocampal-LO connectivity was significantly greater than the correlation between PFC-LO connectivity and associative memory (Hotelling-William Test, t25 = 2.65, P < .02). These data highlight the specificity of the hippocampal-cortical correlations in particular as being critical for memory strengthening.
In addition to assessing zero-lag correlations, we computed cross-correlation functions to more fully examine the dynamics between ROI pairs. As expected based on our initial correlation results, significantly higher cross-correlation values were found for Post-OF versus Baseline Rest for several seconds surrounding a lag of 0 seconds (Figures S2, S3). However, other differences in the shapes of the cross-correlation functions were also evident. To more fully characterize these differences, we compared the magnitude of the coherence between ROI pairs for the Post-OF and Baseline Rest periods. Significantly greater coherence was found for the right FFA-LO and right FFA-PPA for Post-OF compared to Baseline Rest in the frequency ranges of .02 - .06 Hz and .04 - .05 Hz, respectively (Figure S2). This analysis indicates that correlated fluctuations in the BOLD signal at a frequency range of less than .1 Hz are related to previous experience. Furthermore, these results suggest that higher frequencies, which can be preferentially contaminated by cardiac and respiratory activity (Cordes et al., 2001), do not contribute to our findings.
Finally, to address the dynamics of these changes over time, we asked whether the observed enhancement in ROI correlations during Post-OF Rest is evident initially and then decays over time (as suggested by decreases in reactivation in rodent studies, Wilson and McNaughton, 1994). Specifically, we examined the consistency of the enhanced correlations over the course of the entire 8.4 minute rest scan in 43.75 s sliding windows (see Experimental Procedures). Notably, the correlations computed on this local time scale were also significantly greater for the Post-OF Rest versus the Baseline rest for the right FFA-LO (t13 = 3.63, P < .004), right FFA-PPA (t15 = 3.28, P < .006), and hippocampal-LO correlations (t13 = 3.01, P < .02). We performed a linear regression of the average correlation across subjects and time in the Baseline and Post-OF Rest scans and found that the difference between correlations in the Post-OF Rest and the Baseline rest did not exhibit a linear change over time (Figure S2; FFA-LO, r = -.07, P > .5; FFAPPA, r = -.20, P > .1), although a trend for a decrease over time was found for the hippocampal-LO correlation (Figure S3; r = -.21, P < .09). These findings indicate that enhanced correlations present in the Post-OF Rest were generally consistent and present throughout the entire rest scan. However, future studies incorporating longer rest scans will be needed to fully characterize any changes in correlations over time.
DISCUSSION
In conclusion, we provide evidence that experience-dependent changes in BOLD fluctuations during rest are related to subsequent memory for pre-rest experiences. These novel findings strongly support two predictions of memory consolidation theories (Marr, 1971; McClelland et al., 1995; Nadel et al., 2000; Squire et al., 1984). First, enhanced hippocampal-cortical (in the present case; hippocampal-LO) interactions were found during rest following an experience with high levels of later associative memory and the magnitude of resting correlations across subjects predicts individual differences in later associative memory for the preceding experience. Second, enhanced cortico-cortical interactions also were found during rest, depending on the later strength of memory for that experience. These findings are consistent with work in rodents showing that coordinated hippocampal-cortical activity occurs during sleep (Ji and Wilson, 2007; Peyrache et al., 2009; Qin et al., 1997; Siapas and Wilson, 1998; Wierzynski et al., 2009). Importantly, however, the present data extend these findings by demonstrating that coordinated hippocampal-cortical activity also occurs during awake rest in humans, and that these interactions during rest have implications for later memory. Finally, these results complement a recent finding that the magnitude of human hippocampal activation during rest is related to trait-level measures of memory (Wig et al., 2008) by showing that resting correlations also predict memory specifically for recent pre-rest experiences.
An alternative explanation for strong post-task resting correlations is that they may be the direct consequence of strong encoding activation previously shown to predict later memory performance (Brewer et al., 1998; Wagner et al., 1998; for a review see Davachi, 2006), that is maintained during subsequent rest. However, our data argue against this interpretation by showing that enhanced correlations during rest do not merely reflect, or mirror, previously induced correlations during behavior. Differential patterns of FFA-PPA connectivity were found across Object-Face and Scene-Face Encoding and Post-OF and Post-SF Rest, as shown by a significant interaction between Task (Object-Face/Scene-Face) and Time Period (Encoding/Rest) (Figure 2C-D). Specifically, enhanced resting correlations were found between the right FFA and PPA during Post-OF Rest only, despite greater FFA-PPA correlations during Scene-Face vs. Object-Face Encoding. Given the greater subsequent associative memory for object-face pairs compared to scene-face pairs, these results suggest that patterns of functional connectivity between brain areas may be more related to future memory than to the patterns of task-induced activity.
In this study, we found evidence for enhanced hippocampal-cortical (hippocampal-LO) and cortico-cortical connectivity during rest following a condition with relatively high levels of subsequent memory (Object-Face Encoding) but not after an experience with relatively lower levels of subsequent associative memory (Scene-Face Encoding). The difference in associative memory across the two encoding tasks may be due to the specific stimuli chosen for Scene-Face Encoding. Specifically, the scene stimuli used in scene-face pairs contained scenes drawn from one of eight semantic categories, which likely resulted in a high degree of semantic overlap between individual scene exemplars. This potential lack of distinctiveness for individual scenes likely made it difficult for subjects to form separable associations between particular scene-face pairs, whereas distinctive objects seen during Object-Face Encoding may have allowed for more unique associations between object and face stimuli; these differences may have resulted in better associative memory for object-face versus scene-face pairs. It will be essential for future studies to fully explore the relationship between differences in subsequent associative memory and enhanced connectivity during post-encoding rest.
The present results complement a series of recent findings that have related reactivation in rodents to behavioral learning experiences. In previous studies, replay has typically been reported after animals perform highly familiar, repetitive tasks (Diba and Buzsaki, 2007; Foster and Wilson, 2006; Lee and Wilson, 2002) during which it is unclear how much novel learning is taking place, begging the question of how much reactivation is related to memory formation per se. However, very recent work has revealed a direct relationship between changes in learning performance and modifications in the amount of later reactivation (Ego-Stengel and Wilson, 2009; Girardeau et al., 2009; Nakashiba et al., 2009). Additionally, these results are supported by findings that have related novel learning experiences to changes in patterns of neural activity during off-line periods (Cheng and Frank, 2008; Peyrache et al., 2009; Ribeiro et al., 2004; Ribeiro et al., 2007; Tatsuno et al., 2006) and changes at the level of the local field potential during sleep (Clemens et al., 2005; Eschenko et al., 2006; Eschenko et al., 2008; Gais et al., 2002). Here we show that the magnitude of post-task interregional correlations following the encoding of novel stimulus pairs are related to how well the associations between those pairs are later remembered, highlighting the relationship between off-line interactions and memory consolidation.
Over the past decade, there has been substantial interest in measuring resting state correlations in humans in order to reveal anatomical networks, to characterize abnormalities in disease states, as well as for other applications (Fox and Raichle, 2007). However, the functional role of resting state connectivity is still unclear (Buckner and Carroll, 2007). The present findings that resting connectivity is influenced by recent experiences are concordant with two related but distinct studies that ask if connectivity in the ‘default network’ (Raichle et al., 2001), per se, changes based on previous experiences (Albert et al., 2009; Hasson et al., 2009). Specifically, Hasson et al. (2009) showed that correlations between ‘default network’ regions can be modulated during task and rest periods by the prior task content, and found that lower levels of network connectivity during task are related to better comprehension of information during the task. Furthermore, Albert et al. (2009) found that connectivity in the default network, as well as in the cerebellum, is specifically enhanced during rest after a task requiring motor learning, demonstrating a relationship between learning and changes in resting connectivity, similar to changes seen in rodents (Eschenko et al., 2006; Eschenko et al., 2008; Peyrache et al., 2009; Ribeiro et al., 2004). The current results extend this work by showing that connectivity between regions engaged during an encoding task are later modulated during rest and this modulation in resting connectivity is related to subsequent memory for task elements. Taken together, these findings provide new insight into the functional significance of resting state correlations by illustrating their potential importance for memory consolidation.
In the present study, each subject's memory was tested a relatively short time after the scanned encoding session. Specifically, the memory test began approximately 40-50 minutes and 70-80 minutes after the second and first encoding tasks, respectively. Although it is likely that changes in protein synthesis underlying intermediate forms of long-term potentiation occur in this time frame (Reymann and Frey, 2007), it is unclear if this delay is long enough to allow for systems level memory consolidation to begin. It will be essential for future studies to assess how connectivity during post-task off-line periods relates to more extended measures of long-term memory consolidation. Furthermore, it will be interesting to explore the relationship between longitudinal measurements of enhanced connectivity and behavioral measures of memory consolidation.
One question when examining memory and resting state-related activity in humans is whether active rehearsal of stimuli occurs during rest periods. We think this is unlikely to contribute to our results for several reasons. First, there were no task demands to encourage rehearsal because subjects were not informed of the memory test until after the scanning session. Second, in a post-study questionnaire, no subjects reported thinking about any of the preceding stimuli during any of the rest scans. Finally, if active rehearsal during rest is the basis for the present findings, we might expect that correlations between PFC and posterior cortical regions (such as LO) would show enhanced correlation during post-task rest that predicts later memory performance (Davachi et al., 2001). However, post-task PFC-LO correlations did not predict memory performance differences across individual subjects, suggesting that associative memory performance in this experiment was not modulated by active rehearsal processes.
This work adds to a growing body of literature highlighting the importance of coordinated brain activity during off-line rest periods for memory consolidation. Recent work in rodents has shown that patterns of activity in the hippocampus that represent a behavior are reactivated during awake rest (Diba and Buzsaki, 2007; Foster and Wilson, 2006; Karlsson and Frank, 2009). Here, we extend this result to humans by showing that the strength of resting BOLD correlations varies with the extent to which experiences are later remembered. On average, BOLD correlations were enhanced following experiences with high later associative memory and, furthermore, the magnitude of resting correlations predicted individual variability in later associative memory. Taken together, these results provide strong evidence that resting brain correlations contribute to long-term memory and suggest that may be pivotal in facilitating memory consolidation.
EXPERIMENTAL PROCEDURES
Subjects
Sixteen right-handed native English speakers with normal or corrected-to-normal vision participated in the study (nine male, seven female). Subjects’ ages ranged from 22-34 with a mean of 27.4 years. Informed consent was obtained from all subjects in a manner approved by the Institutional Review Board at New York University.
Procedure
All subjects performed a rest scan (Baseline Rest), followed by a task (Object-Face or Scene-Face Encoding), a second rest scan (Post-OF or Post-SF Rest), a different task (Scene-Face or Object-Face Encoding), and a third rest scan (Post-SF or Post-OF Rest), and three localizer scans. The order of the Object-Face and Scene-Face Encoding tasks was counterbalanced across subjects. A high resolution anatomical scan was collected for each subject after the localizer scans. Following the completion of the scanning session, subjects were administered a surprise memory test outside the scanner. Subjects were not informed prior to the scanning session that their memory for stimuli in the encoding tasks would be tested. Finally, after the memory test, subjects filled out a questionnaire that asked what they thought about during the rest scan. Subjects were explicitly asked to categorize what percent of time in the rest scans they thought about stimuli present in the encoding tasks and to categorize what else they thought about during the rest scans. All subjects performed a brief practice session for the localizer task and Object-Face Encoding task prior to entering the scanner.
Encoding tasks
Subjects performed both Object-Face and Scene-Face Encoding tasks, during separate blocks. Each task was run over two 10.5 minute scans. Each scan contained 36 trials for a total of 72 trials in both encoding tasks. Each trial lasted for 17.5 s and consisted of a fixation cue for 1 s, presentation of an object-face or scene-face pair for 5 s, followed by a response period for 1s, and a baseline “arrows” task for 10.5 s between trials (Figure 1A; (Stark and Squire, 2001). Stimulus pairs were presented with one stimulus to the left of fixation and the other stimulus to the right of fixation (Figure 1A). During the response period, subjects responded to the prompt ‘Likely or Unlikely?’ for the Object-Face Encoding task and ‘Happy or Unhappy?’ for the Scene-Face Encoding task. For the arrows task, subjects were presented with an arrow randomly pointing to the left or right, and were instructed to press their middle finger of their left hand when the arrow was pointing to the left and their index finger of their left hand when the arrow was pointing to the right.
For the Object-Face Encoding task, subjects were instructed to vividly imagine the person pictured manipulating the object and then to decide if it was likely or unlikely for this person to be manipulating this particular object. Subjects indicated their response by pressing the middle finger of their left hand for likely and the index finger of their left hand for unlikely. For the Scene-Face Encoding task, subjects were instructed to vividly imagine the person in the environment pictured. Based on this mental image, subjects were instructed to decide if the particular person would be happy or unhappy in the paired environment. These tasks were chosen to require subjects to not only attend to and process each stimulus in the pair but to also attend to the association of the two stimuli.
For both the Object-Face and the Scene-Face Encoding tasks, half of the trials consisted of pairs with male faces and the other half with female faces (36 for each gender). The left/right orientation of the stimuli was counterbalanced such that half of the male face and female face pairs contained the face to the left of fixation (18 male, 18 female), and the other half of trials contained the face to the right of fixation (18 male, 18 female).
Memory test
All subjects performed a memory test outside of the scanner after completing the scanning session. Separate tests were administered for the Object-Face and Scene-Face tasks; the order of memory tests matched the order that they were performed during the scanning session. Each memory test was self-paced and consisted of 96 trials. Similar to the encoding tasks, each trial in the memory test consisted of an object-face or a scene-face pair with one stimulus to the left and to the right of fixation. Each stimulus pair was presented on the computer screen and subjects were instructed to rate the pair as either ‘intact’, ‘rearranged’, or ‘new’ (the cue ‘Intact, Rearranged, or New?’ appeared on the screen below the stimuli). Subjects were instructed to respond ‘intact’ if they remembered that both stimuli in the pair were presented during encoding and that the stimuli were presented together. If the subjects remembered encountering both stimuli during encoding but did not think that they were presented together, they were instructed to press ‘rearranged’. However, if subjects thought that one or both of the stimuli in the pair were novel (not shown during encoding), they were instructed to press ‘new’. When subjects reported stimuli as ‘new’, they were further probed to determine if they thought the face, object or scene, or both stimuli were new (the cue ‘Face, Object, or Both?’ or ‘Face, Scene, or Both?’ appeared below the stimuli).
The majority of trials in the memory test consisted of intact and rearranged trials, as the main behavioral measure of interest was associative memory for the stimulus pairs. Half of the trials were intact pairs (48), one-sixth were rearranged (16), and one-third were new (32). Of the new trials, half contained two new stimuli (16), a quarter consisted of a new face and an old object or scene (8), and another quarter contained an old face with a new object or scene (8). The gender and left/right orientation of faces was counterbalanced across all trial types.
In order to evaluate associative memory for stimulus pairs, we calculated an associative memory measure of associative hits minus associative misses for each subject. Associative hits were the percent of ‘intact’ trials correctly labeled ‘intact’ and associative misses were the percent of ‘intact’ trials labeled ‘rearranged’. Thus, the associative memory measure is an indicator of how often subjects remembered the stimulus pairs, taking into account how often they accurately recognized both stimuli in the pair without their association.
Stimuli
The face stimuli were drawn from the AR face database (Martinez and Benavente, 1998) and from a face database compiled by Prof. Sverker Sikstroem at Lund University Cognitive Science. Object stimuli were obtained from a CD-ROM database and the scene stimuli were drawn from an online database (http://cvcl.mit.edu/database.htm, Oliva and Torralba, 2001). Scrambled objects were created by dividing images of objects into a 20×20 pixel grid and randomly arranging the location of each 20×20 block in the grid. Separate sets of images were used for the encoding tasks and the localizer scans to ensure that no stimuli used in the localizer scans were also present in the Object-Face and Scene-Face Encoding tasks. Furthermore, no overlapping stimuli were used in the Object-Face and Scene-Face Encoding tasks for each subject, although the stimulus sets were not fixed across subjects. No new stimuli in the memory test were stimuli used in the localizer scans or in any of the other tasks.
Localizer task
A four-category localizer was used to define regions involved in processing the stimuli present in the Object-Face and Scene-Face Encoding tasks. The localizer scan consisted of blocks of fixation (14 s) and blocks of viewing of objects, faces, scenes, and scrambled objects. Each block was 14 s in duration and contained 20 stimuli presented for 300 ms each, followed by an inter-stimulus interval of 400 ms. During the scan, subjects were instructed to pay attention to the stimuli as they were presented and to indicate (via pressing the index finger of their left hand) when they noticed a stimulus presented twice in a row (a 1-back task). Two repeats occurred in each block. Each localizer scan lasted for 5 minutes and consisted of five fixation blocks and four blocks of each of the stimulus categories. The order of the stimulus blocks was counterbalanced within and across scans. Each subject performed three localizer scans for a total of 12 blocks of each stimulus type and 15 fixation blocks.
Rest Scans
During the rest scans, subjects were instructed to close their eyes and simply think about anything that they wanted, but to remain awake (Damoiseaux et al., 2006; Greicius et al., 2003). Each rest scan lasted for 8.4 minutes.
fMRI Data Acquisition and Preprocessing
Scanning was performed using a 3T Siemens Allegra MRI system with a whole-head coil. Functional data were collected using a gradient-echo planar pulse (EPI) sequence (repetition time = 1.75 s, echo time = 30 ms, field of view = 192 mm, 31 slices oriented AC-PC, 3 × 3 × 3 mm voxel size, .6 mm interslice gap, 288, 360, and 172 volumes for the rest, encoding task, and localizer task runs). High resolution T1-weighted (magnetization-prepared rapid-acquisition gradient echo) images were acquired for anatomical visualization. Visual stimuli were projected onto a screen that was viewed through a mirror attached to the subject's head coil.
The imaging data were preprocessed using SPM5 (Wellcome Department of Cognitive Neuroscience, University College London, London, UK). The data was first corrected for differences in slice acquisition timing, followed by motion correction across runs. For the definition and analysis of perceptual ROIs (FFA, LO, and PPA), the functional data remained in subject-specific space and were spatially smoothed with a 6 mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. To define ROIs in a group level analysis (hippocampal and prefrontal ROIs), the functional and anatomical data were spatially normalized to an EPI template in Montreal Neurological Institute (MNI) space. After this transformation, the functional data were spatially smoothed with a 6 mm FWHM isotropic Gaussian kernel. Low frequencies (less than 2 cycles per run) and linear trends were removed from the functional data in the localizer and encoding tasks.
fMRI Analysis
ROI Definition
Based on the localizer scans, the fusiform face area (FFA), the posterior portion of the lateral occipital complex (LO), and the parahippocampal place area (PPA) were defined on each individual subject. The FFA was defined as a region in the fusiform gyrus that responded more to faces than objects (P < 10-4, Kanwisher et al., 1997), LO as regions in the posterior occipital cortex that responded more strongly to objects than scrambled objects (P < 10-4, Grill-Spector et al., 2001; Malach et al., 1995), and the PPA as a region in the posterior parahippocampal cortex which responded more to scenes than objects and faces (a conjunction of scenes greater than objects and scenes greater than faces, P < 10-4, Epstein & Kanwisher, 1998). The right FFA and right and left PPA were defined in all 16 subjects; however, the left FFA and LO bilaterally could not be defined in two subjects.
To define ROIs specifically related to associative memory formation for stimuli in the encoding tasks, a subsequent memory analysis was performed. This analysis was performed on the normalized functional data at the group level. In order to probe regions of the brain with a greater BOLD response at encoding for stimulus pairs with intact associative memory, we performed a general linear model with regressors for all trial types based on subjects’ responses during the memory test for both the Object-Face and Scene-Face Encoding tasks. The contrast of encoding trials correctly later labeled ‘intact’ greater than encoding trials later incorrectly labeled ‘rearranged’ revealed activations in left posterior hippocampus and left lateral prefrontal cortex. Hippocampal and prefrontal ROIs were defined as at least six contiguous voxels significant at P < .005 and P < .001, respectively. A reduced threshold was used for the hippocampal ROI to compensate for reduced signal to noise ratio in medial temporal lobe regions (Davachi and Wagner, 2002; Duncan et al., 2009; Ojemann et al., 1997; Preston and Gabrieli, 2008; Schacter and Wagner, 1999; Strange et al., 2002; Weis et al., 2004).
Rest Data Processing and Analysis
In order to examine the correlation in the BOLD signal across predefined regions of interest, we extracted the time course for each ROI (FFA, LO, PPA, hippocampal, and prefrontal ROIs) for the three rest scans in each individual subject. Low frequency trends were removed using a high-pass filter with a cutoff of .009 Hz, which has been used in previous studies examining functional connectivity at rest (Fox et al., 2006; Fox et al., 2005; Vincent et al., 2006). Pearson correlation coefficients were then computed between the BOLD time course of ROI pairs in each rest run (Baseline, Post-OF, and Post-SF Rests) for each subject. All statistics were computed on Fisher Z-transformed correlation (r) values. T-tests were then used to compare the magnitude of the correlation for a given ROI pair across rest scans (e.g., between Baseline and Post-OF Rest) by comparing the Z-transformed data across runs.
In addition to parametric tests, all differences in correlations between rest scans were confirmed via two non-parametric tests. First, a non-parametric test was performed in which the correlation (r) values for all subjects for the two rest scans of interest were concatenated and the difference between the mean of two random partitions of the data set was calculated (Maris et al., 2007). This process of randomly partitioning the correlation values and calculating the statistic of interest (the difference between the mean correlation of the two groups) was then repeated 10,000 times to generate a null distribution of the mean correlation difference for each comparison between rest scans. Second, a randomization test was performed in which the time courses for each ROI pair were concatenated across subjects, and the subject labels were randomly assigned. The correlation between time courses in the two ROIs was then performed for the mislabeled data and the difference between the mean correlation of the two rest scans of interest was calculated. This process was repeated 10,000 times to generate another null distribution of the mean correlation difference between rest scans. The true difference between the mean correlation of the two rest scans was then compared to these null distributions to determine the level of significance (shown in Figures S2, S3).
To evaluate relationships between ROI pair correlations during rest and subsequent associative memory performance, we computed Pearson correlation coefficients between associative memory performance and Z-transformed correlation coefficients across individual subjects. In order to evaluate whether there are significant differences between different ROI pairs’ correlations with behavior (e.g., looking for differences in the extent to which Hippocampal-LO and PFC-LO correlations correlate with memory performance), a Hotelling-William test for comparing dependent correlations was used (Steiger, 1980).
In order to determine if correlations between ROI pairs vary throughout the course of the rest scan, correlations were calculated on a smaller time scale to examine linear trends in correlation values over time. Specifically, correlations between ROI pairs were calculated in 43.75 s blocks (25 TRs), stepping through the entire 8.4 minute rest scan in intervals of 7 s (4 TRs). A linear regression was performed between time in the rest scan and the average correlation for ROI pairs across subjects.
To examine the dynamics of interactions between ROIs, we calculated the coherence between ROI pairs based on multi-taper spectral estimates with .014 Hz resolution (http://chronux.org/, Mitra & Bokil, 2008). In order to compare differences in the coherence between rest scans, the magnitude of the coherence was Z-transformed according to (Jarvis and Mitra, 2001) based on the degrees of freedom in the estimator. Specifically, the Z-transformation was calculated as z = β (q − β) where , C is the coherency, ν is the degrees of freedom, and β is a parameter that was fitted independently. Since the coherence was calculated for each rest run in each subject, ν is equal to the two times the number of tapers used to estimate the coherence (13 tapers were used). The beta parameter was determined from the Post-SF Rest data (the value of β which resulted in a coherence with a variance of approximately 1), as our primary interest was in comparing the coherence between the Baseline and Post-OF Rest periods (β = 1.01 for FFA-LO and FFA-PPA coherence and β = 1.13 for hippocampal-LO coherence). We then evaluated the significance of the difference in coherence between the Baseline and Post-OF Rest periods between .01 and .1 Hz as frequencies above .1 Hz are known to be influenced by respiratory and cardiac activity (Cordes et al., 2001) and the frequencies below .009 Hz were filtered during preprocessing. We then calculated the average Z-transformed coherence for each subject in frequency bins centered at .015 Hz though .095 Hz in intervals of .01 Hz, with ± .005 Hz windows (i.e., bins of .01-.02, .02-.03, ..., .08-.09, and .09-.1 Hz). T-tests were performed at each frequency bin and were corrected for multiple comparisons. Confidence intervals for the coherence were constructed by bootstrapping across tapers and individual subjects (shown in Figures S2, S3).
Supplementary Material
ACKNOWLEDGEMENTS
We thank K. Oschner and K. Duncan for suggestions on earlier versions of the manuscript and B. Pesaran for advice regarding the coherence analyses. This work was supported by National Institute of Mental Health Grant MH074692 and Dart Neuroscience to L.D.
REFERENCES
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cheng S, Frank LM. New experiences enhance coordinated neural activity in the hippocampus. Neuron. 2008;57:303–313. doi: 10.1016/j.neuron.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Maril A, Wagner AD. When keeping in mind supports later bringing to mind: neural markers of phonological rehearsal predict subsequent remembering. J Cogn Neurosci. 2001;13:1059–1070. doi: 10.1162/089892901753294356. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Curtis C, Davachi L. Distinct memory signatures in the hippocampus: intentional States distinguish match and mismatch enhancement signals. J Neurosci. 2009;29:131–139. doi: 10.1523/JNEUROSCI.2998-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2009 doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Molle M, Born J, Sara SJ. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006;26:12914–12920. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Ramadan W, Molle M, Born J, Sara SJ. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn Mem. 2008;15:222–228. doi: 10.1101/lm.726008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci U S A. 2009;106:10841–10846. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science. 2002;297:2070–2073. doi: 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput. 2001;13:717–749. doi: 10.1162/089976601300014312. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci U S A. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Schoffelen JM, Fries P. Nonparametric statistical testing of coherence differences. J Neurosci Methods. 2007;163:161–175. doi: 10.1016/j.jneumeth.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Martinez AM, Benavente R. The AR Face Database. In CVC Tech. Report. 1998 [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10:352–368. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron. 2009;62:781–787. doi: 10.1016/j.neuron.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci. 2009;12:919–926. doi: 10.1038/nn.2337. [DOI] [PubMed] [Google Scholar]
- Preston AR, Gabrieli JD. Dissociation between explicit memory and configural memory in the human medial temporal lobe. Cereb Cortex. 2008;18:2192–2207. doi: 10.1093/cercor/bhm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin YL, McNaughton BL, Skaggs WE, Barnes CA. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Philos Trans R Soc Lond B Biol Sci. 1997;352:1525–1533. doi: 10.1098/rstb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Born J. Maintaining memories by reactivation. Curr Opin Neurobiol. 2007;17:698–703. doi: 10.1016/j.conb.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Gervasoni D, Soares ES, Zhou Y, Lin SC, Pantoja J, Lavine M, Nicolelis MA. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol. 2004;2:E24. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Shi X, Engelhard M, Zhou Y, Zhang H, Gervasoni D, Lin SC, Wada K, Lemos NA, Nicolelis MA. Novel Experience Induces Persistent Sleep-Dependent Plasticity in the Cortex but not in the Hippocampus. Front Neurosci. 2007;1:43–55. doi: 10.3389/neuro.01.1.1.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Squire LR, Cohen NJ, Nadel L. The medial temporal region and memory consolidation: a new hypothesis. In: Weingartner G, Parker E, editors. Memory Consolidation. Erlbaum; Hillsdale, NJ: 1984. pp. 185–210. [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci. 2002;22:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10:180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Tatsuno M, Lipa P, McNaughton BL. Methodological considerations on the use of template matching to study long-lasting memory trace replay. J Neurosci. 2006;26:10727–10742. doi: 10.1523/JNEUROSCI.3317-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G. Neural correlates of successful declarative memory formation and retrieval: the anatomical overlap. Cortex. 2004;40:200–202. doi: 10.1016/s0010-9452(08)70950-x. [DOI] [PubMed] [Google Scholar]
- Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61:587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Wolford GL, Petersen SE, Kelley WM. Medial temporal lobe BOLD activity at rest predicts individual differences in memory ability in healthy young adults. Proc Natl Acad Sci U S A. 2008;105:18555–18560. doi: 10.1073/pnas.0804546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.