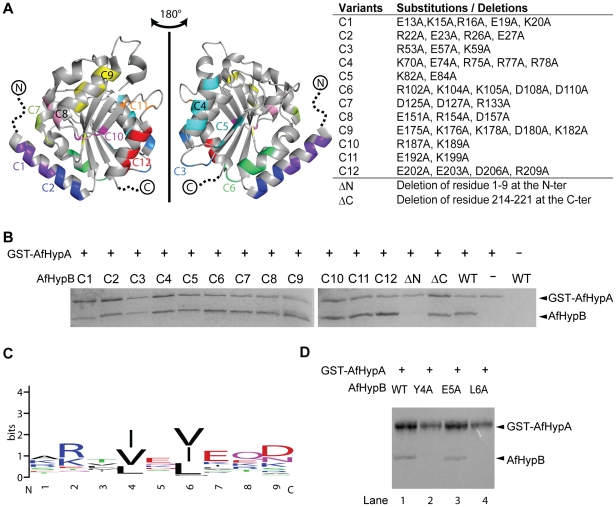
Figure 2. The unstructured N-terminal residues of AfHypB is required for HypA/HypB interaction.
(A) Design of variants to map the HypA binding site on HypB. Twelve clustered-charge-to-alanine variants designated C1–C12, were created according to the close proximity of the charge residues in the crystal structure of AfHypB (PDB code: 2WSM). Residues 1–9 and 214–221 were unstructured in AfHypB. Two truncation variants, ΔN and ΔC, were created to investigate the role of these unstructured residues. (B) Interaction of AfHypB variants with AfHypA was tested by in vitro pull-down assay. Cell lysate containing GST-AfHypA and HypB variants was loaded on to glutathione-resin. Bound proteins were eluted by 20 mM glutathione after extensive washing. All the HypB surface charge mutants and C-terminal mutant were co-eluted with HypA suggesting that interaction was not disrupted by the mutations. N-terminal truncated HypB mutant was incapable to be co-eluted with HypA indicating that the first nine amino acid residues of AfHypB is required to interact with HypA. (D) Residues 4 and 6 (numbering according to the sequence of AfHypB) are conserved among HypB. The sequence logo representation was generated by the program WEBLOGO [35], [36] using 62 non-redundant (with <90% identity) sequences from the NCBI non-redundant database. Aliphatic hydrophobic residues are always found at these two positions. (E) Interaction of AfHypB variants Y4A, E5A and L6A with AfHypA was tested by in vitro pull-down assay. The variant E5A (lane 3) retained its interaction with AfHypA like the wild-type AfHypB (lane 1). Y4A (lane 2) and L6A (lane 4) variants were incapable to interact with AfHypA indicating that both residues are required to interact with HypA.