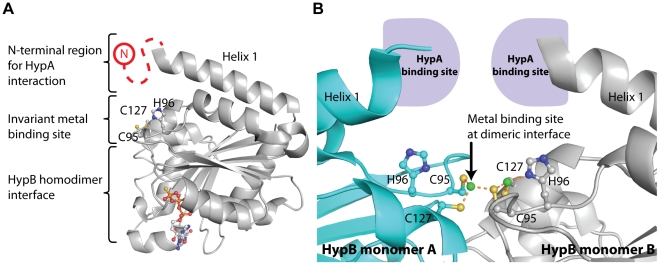
Figure 5. Close proximity of the HypA and metal binding sites of HypB suggests possible metal transfer between HypA and HypB.
(A) The N-terminal residues of HypB, responsible for HypA binding, were not resolved in the crystal structures of HypB (red dashed line). HypB contains an invariant metal binding site composed of two cysteine residues and one histidine residue (C95, H96 and C127 in M. jannaschii HypB, PDB code: 2HF8). The close proximity between the metal binding site at the dimeric interface and the HypA binding site at the N-terminus of the GTPase domain suggest that HypA/HypB interaction may facilitate transfer of nickel between the two proteins. (B) Binding of HypA to HypB may facilitate transfer of nickel between HypA and the metal binding sites (C95, H96 and C127 in M. jannaschii HypB) situated at the dimeric interface of HypB. This metal binding site at the dimeric interface was occupied by zinc (green). Dimerization of HypB may induce steric hinderance between the HypA binding sites and prevent HypA from interacting with the dimeric form of HypB.