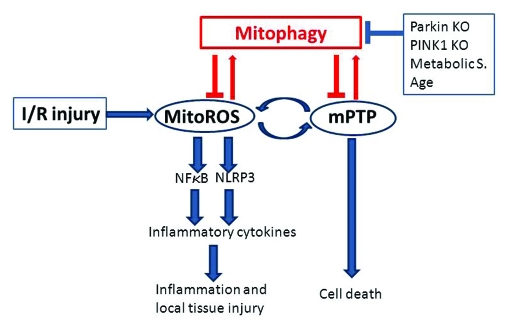
Figure 1.
Connection between mitophagy and inflammation in the heart. Ischemia/reperfusion injury triggers a burst of mitochondrial ROS followed by opening of the mPTP. Depolarization triggers autophagic removal (mitophagy), unless autophagy/mitophagy is impaired [as is the case in the Park2 (Parkin) and Pink1 knockout animals, or in the setting of metabolic syndrome (Metabolic S.) or advanced age]. Failure to eliminate damaged mitochondria leads to a vicious cycle of ROS-induced ROS release, which can trigger mPTP in many mitochondria (catastrophic mPTP), eventually culminating in cell death. ROS derived from mitochondria (mitoROS) activate NFκB, driving transcription of mRNA for inflammatory cytokines, and simultaneously activate the NLRP3 inflammasome responsible for processing the mature forms of IL-1β and IL-18. Released cytokines trigger inflammation and local tissue injury affecting adjacent “innocent bystander” cells. Mitophagy, by promptly eliminating damaged mitochondria, can prevent cell death and inflammatory signaling.