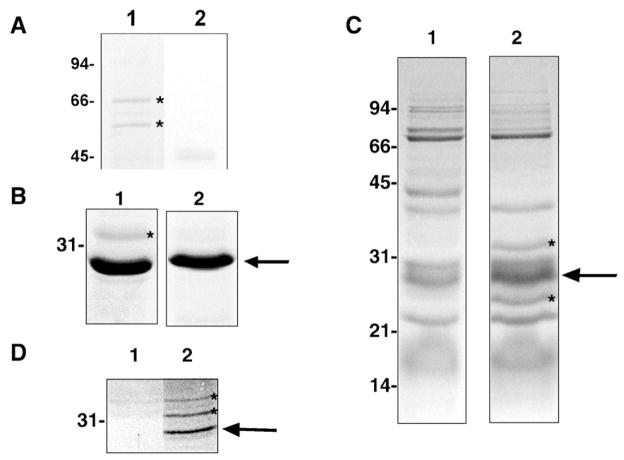
Fig. 1.
Representative findings from affinity chromatography approaches. (A) Co-purifying proteins with DmsD preparation (lane 1; lane 2. control protein preparation). Proteins with asterisk confirmed to interact with DmsD by far-western (not shown). (B) Potential His6-T7-DmsD-interacting species from anaerobic solubilized membrane prey fractions. Eluted fractions from Ni2+-NTA resin exposed to CHAPS-solubilized E. coli membrane prey fractions. Lane 1, His6-T7-DmsD-immobilized resin; lane 2, His6-T7-TehB control protein immobilized resin. (C) Co-purification experiments demonstrating potential His6-T7-DmsD-interacting species from anaerobic expressed DmsD soluble fractions. Lane 1, MC4100; lane 2, MC4100/pTDMS28. (D) Co-purification experiments demonstrating potential His6-T7-DmsD-interacting species from anaerobic solubilized membranes. Putative interacting proteins are indicated with asterisks. Arrows denote the His6-T7-DmsD or control protein. Molecular mass markers (in kDa) are indicated to the left.