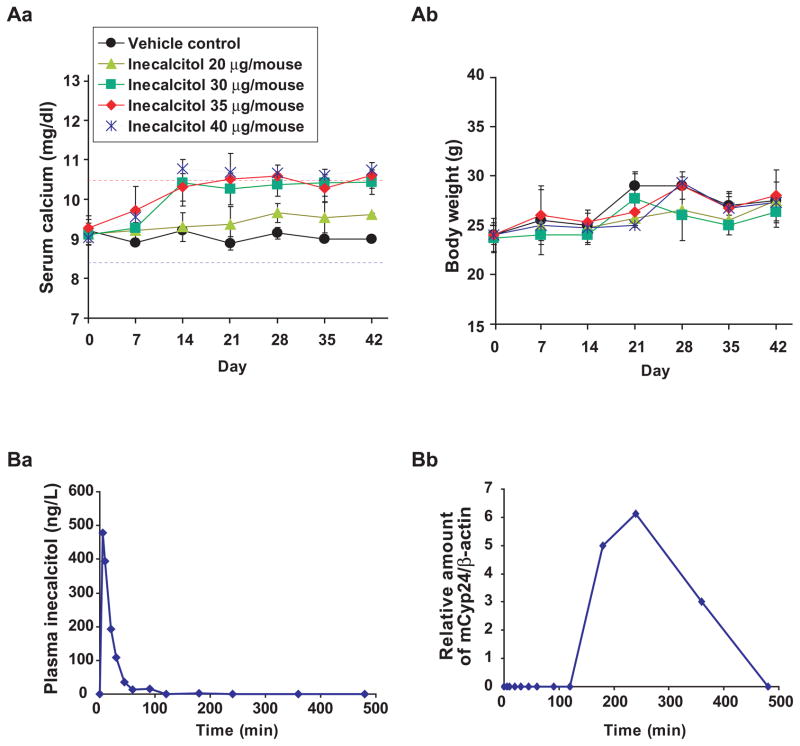
Fig. 3. In vivo administration of inecalcitol: serum calcium, body weights, plasma half-life and Cyp24 levels.
(A) C57BL/6J mice received 20 – 40 μg of inecalcitol three times per week by i.p. for 6 weeks, and serum calcium levels were measured once a week and presented as means and SDs. (Aa) Overall serum calcium levels and (Ab) body weights of the treated mice. ●, vehicle control; ▲, 20;■, 30; ◆, 35; *, 40 μg/mouse of inecalcitol. The MTD (normal calcemic) of inecalcitol by i.p. was 30 μg/mouse (1,300 μg/kg). (B) Pharmacokinetics of inecalcitol in mice (N=3). (Ba) Plots of plasma concentration of inecalcitol and (Bb) mouse Cyp24 (mCyp24) mRNAlevels in liver were measured at indicated time points after injection of inecalcitol (1,300 μg/kg/mouse, i.p.). The results are presented as means (N=3). Plasma calcium levels were in normal range (data not shown) throughout the experiments. Pharmacokinetic studies showed that plasma half-life of inecalcitol was 18.3 minutes. Other pharmacokinetic data are listed in Supplemental Table 3