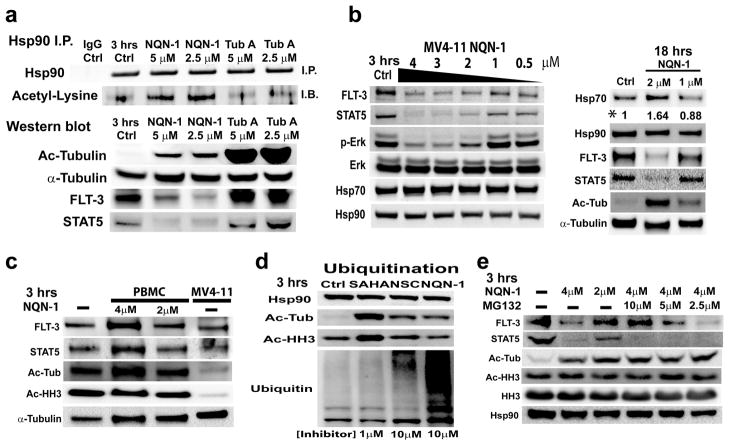
Figure 6. NQN-1 effects on Hsp90 acetylation, mutant FLT-3, STAT5, and Erk phosphorylation.
a) NQN-1 induces Hsp-90 acetylation, and causes degradation of FLT-3, STAT5, and a decrease in the level of phos-Erk 3 hr post inhibitor treatment in MV4-11 cells. b) NQN-1 decreases FLT-3, STAT5, and phos-Erk in a concentration dependent manner. Increase in Hsp70 level was only observed after prolonged treatment, 18 hrs (*quantification with spot-density). c) Tubulin acetylation level did not increase, and normal FLT-3 and STAT5 did not decrease when PBMC was treated with NQN-1. Significantly higher acetylated tubulin and histone H3 levels were observed in PBMC (n=4 normal donors) in comparison to malignant MV4-11 cells. d) NQN-1 and NSC treatment resulted in an increase in total ubiquitinated protein level in MV4-11 cells. e) Proteasome inhibitor, MG132, rescued the depletion of mutant FLT-3 when co-administrated at 10 μM. However, the proteasome inhibitor did not prevent depletion of STAT5 indicating a proteasome independent degradation pathway that is separate from the Hsp90 function.