Abstract
Differential scanning calorimetry was used to study the phase behavior of binary lipid bilayers consisting of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) of varying acyl chain length. A 2-state transition model was used to resolve the individual transition components, and the 2-state transition enthalpy, the relative enthalpy and the transition temperature of each component were plotted as a function of composition. Intriguingly, abrupt changes in these thermodynamic parameters were observed at or close to many “critical” XPE values predicted by the Superlattice model proposing that phospholipids with different headgroups tend to adopt regular rather than random lateral distributions. Statistical analysis indicated that the agreement between the observed and predicted “critical” compositions is highly significant. Accordingly, these data provide strong evidence for that the molecules in PC/PE bilayers tend to adopt regular, superlattice-like lateral arrangements, which could be involved in the regulation of the lipid compositions of biological membranes.
Keywords: regular distribution, membrane, phospholipid, homeostasis, regulation
1. INTRODUCTION
Lateral distribution of the lipids in biological membranes is an active field of study due to its functional implications in many key cellular processes. However, it is very difficult to obtain reliable data on biological membranes due to their highly complex composition. Therefore, many investigators have chosen to study model membranes consisting of only a limited number of lipid species. Apart from the popular raft-model proposing the presence of nanoscopic domains enriched in sphingolipids and cholesterol in cellular membranes,1 we and others have proposed that different lipids tend to adopt regular, superlattice (SL)-like lateral distributions in fluid bilayers and cellular membranes as well.2–4 It is important to note that such regular arrangements should not be considered as permanent or rigid (as implied by drawn models), but rather similar to smectic A′-phases which are highly dynamic and lack long-range order.5 Presumably, the tendency to adopt regular, SL-like distributions is mainly driven by repulsive steric or electrostatic interactions among similar molecules.2 Intriguingly, the “critical” compositions predicted by the SL-model could play a crucial role in the lipid homeostasis of cellular membranes by providing “set-points” regulating homeostatic phospholipases and synthetic enzymes as well.6
Evidence for SL-like distributions in phosphatidylcholine/phosphatidylethanolamine (PC/PE) bilayers has been obtained previously by using fluorescence and FTIR spectroscopy.7–10 In the present study, we used differential scanning calorimetry (DSC) to study the lateral organization of the components in several PC/PE bilayers. Although several DSC studies have been carried out with PC/PE mixtures previously,11–15 the data point composition density in these studies was low thus largely precluding the judgment of the presence of “critical” compositions indicative of SL formation. Accordingly, we varied the composition in very small increments (typically 1 mol%) and then plotted the transition temperature, relative enthalpy and 2-state transition enthalpy of the resolved transition peaks vs. PE mole fraction. Intriguingly, we observed many of the critical compositions predicted by the SL-model in a single PC/PE system, and virtually all of them in the four different PC/PE systems combined.
2. MATERIALS AND METHODS
2.1. Materials
The 1,2-dimyristoyl-sn-glycero-3-PC (DMPC), 1,2-dipalmitoyl-sn-glycero-3-PC (DPPC), 1,2-distearoyl-sn-glycero-3-PE (DSPC), 1,2-dilauroyl-sn-glycero-3-PE (DLPE), 1,2-dimyristoyl-sn-glycero-3-PE (DMPE), 1,2-dipalmitoyl-sn-glycero-3-PE (DPPE), 1,2-distearoyl-sn-glycero-3-PE (DSPE) and 1,2-dielaidoyl-sn-glycero-3-PE (DEPE) species were obtained from Avanti Polar Lipids (Alabaster, Alabama) and were free of impurities as analyzed by thin-layer chromatography. The solvents (LC-grade) were obtained from Merck and the other chemicals from Sigma.
2.2. Preparation of multilamellar liposomes
Calculated amounts of PC and PE dissolved in chloroform/methanol (9:1, v/v) were pipetted with extra care to a glass tube using Hamilton precision syringes equipped with a Cheney adapter. The syringe was chosen based on the volume to be pipetted in order to obtain the accuracy of ± 1 % specified by the pipette manufacturer. This accuracy can also be obtained in practice as tested in our previous studies using mass-spectrometry and deuterium-labeled internal standards.16 After evaporation of the solvent under a nitrogen stream at 35 – 40 °C, the lipids were then dissolved in 0.5 ml of chloroform, and the solvent was again removed under a nitrogen steam at 60 °C. This protocol was used to prevent precipitation of PE before complete evaporation of the solvent, which can occur if methanol is present (unpublished data) as found previously for PC/cholesterol mixtures17. After evaporation of chloroform, the tubes were placed in high vacuum for 6 h to remove any residual solvent. Then 0.5 ml of Tris (10 mM) - EDTA (1 mM) - NaCl (50 mM) buffer, pH 7.0, was added, and the tubes were kept in a 70 °C water bath for 1 min, vortexed and moved to an ice-water bath for 3 min. This heating-cooling cycle was repeated 5 times, and the samples were subsequently stored at 4 °C in the dark.
2.3. DSC measurements
A high-sensitivity 6100 Nano II DSC instrument from Calorimetric Sciences (Lindon, Utah) was used to obtain sequential heating and cooling scans (0.5 °C/min) of samples containing 1 μmol of phospholipid. The scans were repeated for several samples in each set with essentially identical results. A limited set of samples was also analyzed using a heating rate of 0.05 °C/min.
2.4. The multiple 2-state transition model
A DSC apparatus measures the specific heat capacity Cp(T) as a function of temperature T, i.e. the temperature derivative of the total excess molar heat capacity h(T) vs. T.18 The multiple 2-state transition model assumes that the system has n independent transitions and that each of these multiple transitions is characterized by a separate and unique transition temperature (Ti) and an excess molar heat capacity hi(T). If αi(T) denotes the fraction of molecules of the ith component that has completed the transition at the temperature T, the multiple 2-state transition model provides a relationship between hi(T) and αi(T):
| (1) |
where
| (2) |
Here R is the gas constant, ΔH2S,i the 2-state transition enthalpy and ΔHi the enthalpy of the ith independent component. For each ith component, the multiple 2-state transiton model predicts that all molecules in a cooperative unit undergo the phase transition simultaneously, and thus ΔH2S,i represents the collective enthalpy of one cooperative unit. The number of molecules in a cooperative unit (Nc,i) can be estimated by dividing ΔH2S,i by ΔHi (Eq. 3).
| (3) |
The values of Ti, ΔH2S,i and ΔHi for each resolved component were obtained by fitting Eq. 1 to DSC data using nonlinear regression. Fig. 1S in Supporting Information demonstrates the dependence of the αi(T), Cp(T) and Nc,I with ΔH2S,i using simulation. Since the absolute value of ΔHi depends on the quantity of lipid in the sample cell, which may vary slightly, we used a relative value ΔHRel,i as defined by Eq. 4:
| (4) |
The chi-square value (χ2) indicating the goodness of fit was determined from Eq. 5
| (5) |
where yi and ypi are the ith experimental and predicted data points, respectively, while N is the total number of data points and ΔT the temperature increment.
2.5. Deconvolution of resolved thermodynamics parameters versus composition plots
A multiple Gaussian-option of the PeakFit software (Jandel Scientific, San Rafael, CA) was employed to deconvolute the resolved ΔH2S,i or ΔHRel,i according to the multiple 2-state transition model vs. XPE plots. A high frequency filter was applied to assist in fitting so that peaks that are defined by less than three data points were not included in the peak search procedure.8
2.6. SL-model
According to the SL-model the guest molecule can adopt either a hexagonal (HX), centered rectangular (CR) or rectangular (R) distribution in a host lattice of a hexagonal symmetry, and the allowed (“critical”) compositions can be derived from simple geometrical principles.2 For XPE < 0.5 (i.e. when PE is the guest and PC the host), the critical XPE’s for superlattices with a HX, CR or R symmetry are given by Eqs. 5–7:10,19
| (5) |
| (6) |
| (7) |
When XPE > 0.5 (i.e. when PE is the host and PC is the guest), the critical XPE’s are given by Eqs. 8–10:
| (8) |
| (9) |
| (10) |
where a and b indicate the distance in lattice sites between two proximal guest molecules along the principal host lattice axes.3 If the guests form pairs (dimers), the values of the critical XPE’s are twice those for monomeric guests (see Supporting Information).
3. RESULTS
3.1. Calorimetric behavior of PC/PE bilayers
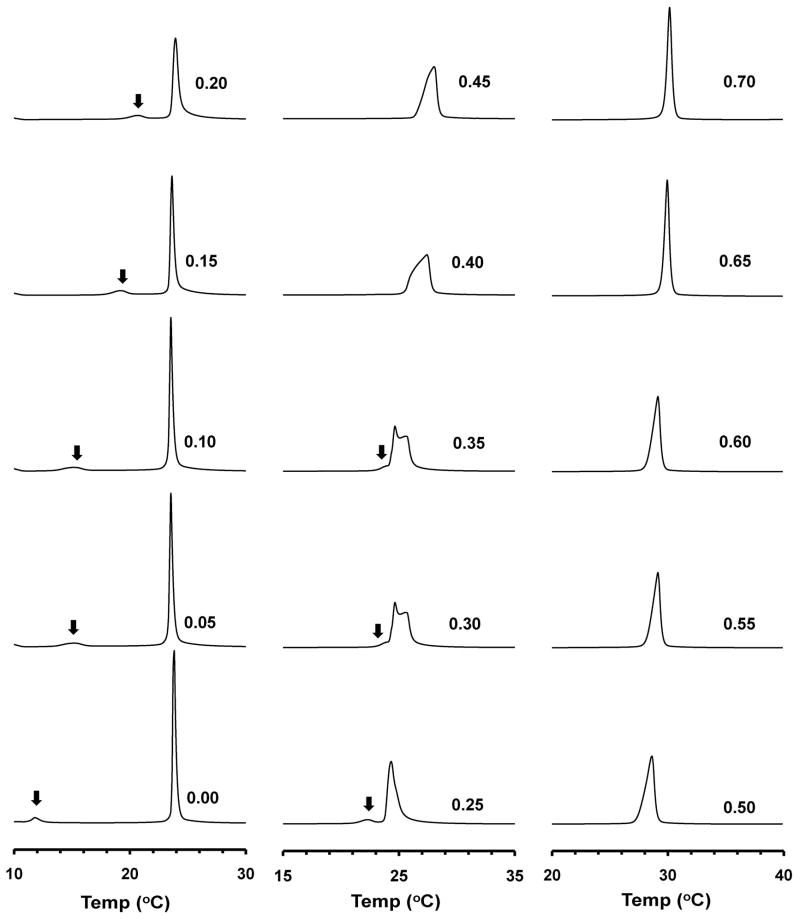
DSC heating scans at a rate of 0.5°C/min were recorded for bilayers consisting of DMPC/DLPE, DMPC/DMPE, DPPC/DMPC, DSPC/DMPE and DSPC/DEPE with XPE varying in 1 mol% increments. Representative scans for DMPC/DLPE bilayers are presented in Fig. 1. For neat DMPC (XPE = 0), the peak temperature of the pre- (Tp) and main (Tm) transitions were found at 11.4 and 23.5 °C, respectively, in agreement with previous data.20 When XPE was gradually increased to 0.30, Tp increased steadily whereas Tm remained relatively constant. The pretransition eventually disappeared or merged with the main transition at XPE of 0.30 – 0.35, while the main transition became broader and eventually developed a pronounced shoulder. Above XPE of 0.40 the main transition narrowed again so that its width at XPE of 0.65 was similar to that at XPE of 0.20. DSC scans for DMPC/DMPE bilayers are presented in Figs. 2SA and 2SB in Supporting Information. The difference between the Tm’s of the PC and PE components in the DMPC/PMPE system is the largest among the PC/PE systems in this study. A similar behavior of merging of Tm/Tp and broadening of the main transition was also detected. No significant and measurable phase separation was detected with the composition range reported in this study.
Figure 1. Representative DSC heating scans for DMPC/DLPE bilayers.
The peak areas have been normalized to ease comparison. Arrowhead indicates the pretransition.
Additional cooling (0.5°C/min) and “slow” heating (0.05°C/min) scans were recorded for a limited number of samples of the different PC/PE mixtures. Fig. S3 in Supporting Information compares data for the heating, cooling and slow scans of DMPC/DLPC bilayers with XPE of 0.29 – 0.38. In general, the shapes, locations and relative enthalpies of the transition components are very similar among the heating, cooling and slower scans for the same XPE. Notably, the lack of significant hysteresis between cooling and heating scans implies that the lateral organization of the bilayer resembles that in the gel and the liquid-crystalline states. The pretransition showed some hysteresis (e.g., for XPE = 0.29 T1 was ~ 20 °C in the cooling scan vs. ~ 23 °C in the heating scan), which is most probably due to the slowness of this transition.21,22
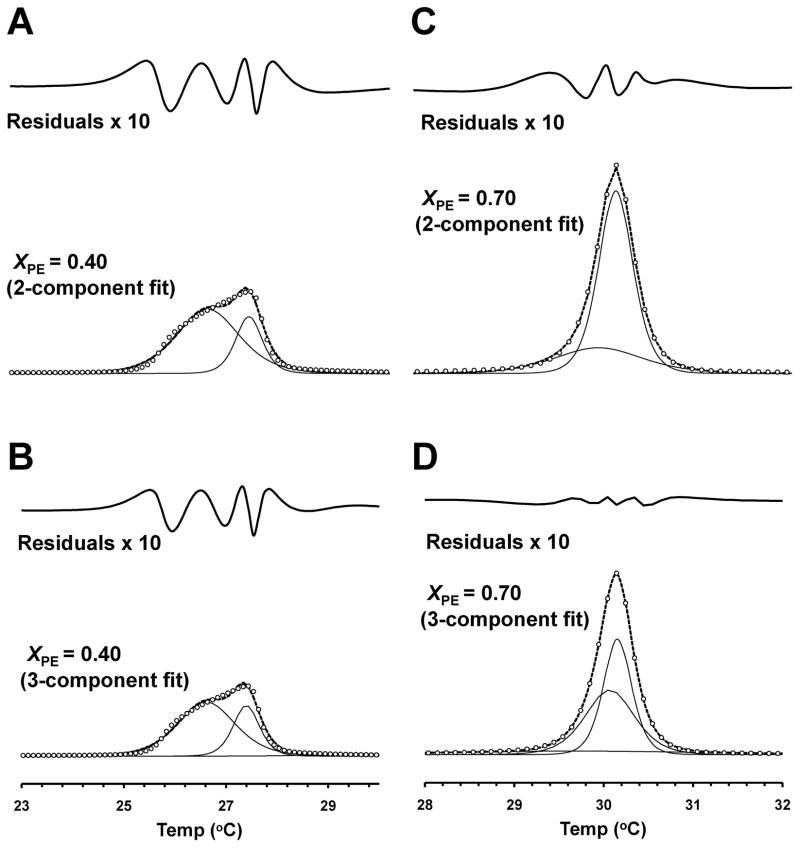
Since the main phase transition of PC/PE bilayers was often asymmetric and in some cases exhibited more than one peak as demonstrated in Fig. 1, a multiple 2-state transition model (see Materials and Methods) was used to resolve the components. Figs. 2 and 3 show examples of 2- and 3-component fits before (XPE = 0 and 0.25) and after (XPE = 0.40 and 0.70) the merge of the pre- and main transitions, while Table 1 summarizes the goodness of fit (χ2) values and the parameters Ti, ΔH2S,i and ΔHRel,i. When the pretransition was detectable (0 < XPE < 0.3), much better fits were obtained by assuming 3 rather than 2 components in total (Fig. 2). In the absence of the (visible) pretransition (i.e. XPE > 0.3), the 3-component model generally did not improve the fit as compared to the 2-component model (Fig. 3), except in a few cases (e.g., χ2 of 0.004 versus 0.014 for XPE = 0.70) as shown in Table 1. This improvement, however, is most probably an artifact due to that the fitting protocol erroneously indicated a curving baseline as a very broad transition. Accordingly, the presence of either two or three transitions overall were assumed in the absence or presence of the pretransition, respectively. For consistency, the parameters for the pretransition are referred to as T1, ΔH2S,1 and ΔHRel,1, while those for the first and second main transition components are referred to as T2, ΔH2S,2 and ΔHRel,2 and T3, ΔH2S,3 and ΔHRel,3, respectively.
Figure 2. Multicomponent fits to DSC data for DMPC/DLPE bilayers in the presence of pretransition.
XPE is 0 (A and B) or 0.25 (C and D). Fits assuming two (A and C) or three (B and D) components are shown. Open circles represent the experimental data points, the solid line the fitted peaks and the dotted line the sum of all fitted peaks. The pre-transition is indicated by an arrowhead. Residuals are shown at the top of each panel.
Figure 3. Multi-component fits to the DSC data for DMPC/DLPE bilayers in the absence of pretransition.
XPE is 0.40 (A and B) or 0.70 (C and D). The 2-state transition peaks based on fits assuming two (A and C) or three (B and D) components are shown. Fitting residuals are shown at the top of each panel.
Table 1.
Representative resolved thermodynamics parameters (Ti, ΔH2s,i, ΔHRel,i) of DMPC/DLPE based on the multi-component 2-state transition models. The relative chisquare (χ2) values of the fits are also shown.
| 2-component Fit | 3-component Fit | |||||||
|---|---|---|---|---|---|---|---|---|
| XPE | Ti (°C) | ΔH2s,i (kcal/mol) | ΔHRel,i (%) | χ2 | Ti (°C) | ΔH2s,i (kcal/mol) | ΔHRel,i (%) | χ2 |
| 0 | 11.83 | 548 | 7.95 | 0.67 | 11.80 | 548 | 7.61 | 0.10 |
| 23.79 | 2075 | 92.05 | 23.75 | 3615 | 38.20 | |||
| 23.97 | 1538 | 54.19 | ||||||
| 0.25 | 22.14 | 604 | 8.16 | 0.43 | 22.17 | 518 | 8.5 | 0.11 |
| 24.37 | 717 | 91.84 | 24.26 | 1116 | 47.35 | |||
| 24.77 | 524 | 44.15 | ||||||
| 0.40 | 26.61 | 462 | 72.80 | 0.04 | 26.58 | 476 | 68.16 | 0.04 |
| 27.37 | 1100 | 27.20 | 27.36 | 1057 | 28.94 | |||
| 29.34 | 384 | 2.90 | ||||||
| 0.70 | 29.91 | 592 | 26.41 | 0.014 | 29.65 | 189 | 12.37 | 0.004 |
| 30.00 | 1515 | 73.59 | 30.25 | 1000 | 43.23 | |||
| 30.11 | 1742 | 44.39 | ||||||
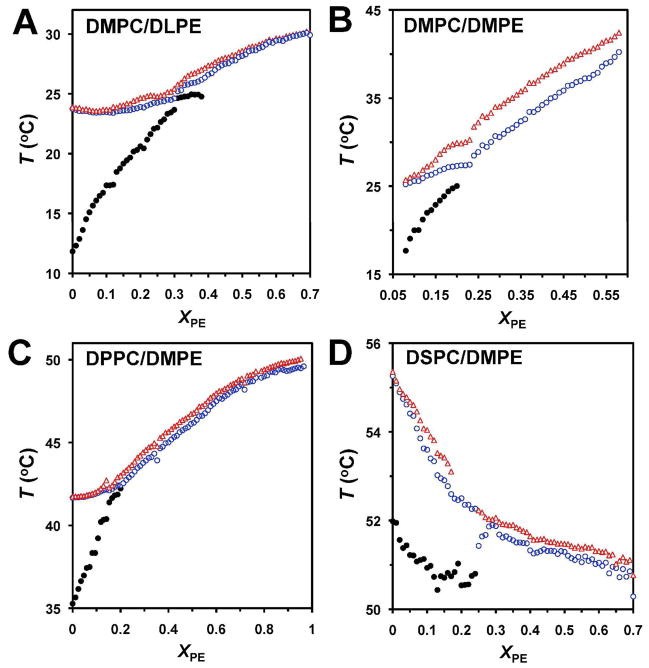
The resolved transition temperatures T1, T2 and T3 obtained from the multiple 2-state transition fits are plotted as a function of XPE for DMPC/DLPE bilayers in Fig. 4A. The T1 plot indicates that the pretransition is present up to XPE of 0.38 – 0.40, thus marking this value as a “critical” one. Other “critical” compositions are indicated by kinks at XPE of ~ 0.13, 0.21 and 0.32. The pretransition was abolished or became undetectable at XPE of ~ 0.20 for both DMPC/DMPE and DPPC/DMPE and ~ 0.25 for DSP/DMPE (Fig. 4B–D) thus marking these compositions as “critical” ones as well. Interestingly, the χ2 values of 2- and 3-component fits showed local minima and merger of the 2- and 3-component fits at or close to predicted critical compositions (see Fig. S4 of Supporting Information).
Figure 4. Peak transition temperatures vs. XPE for different PC/PE bilayers.
(A) DMPC/DLPE, (B) DMPC/DMPE, (C) DPPC/DMPE and (D) DSPC/DMPE. Temperature of the pretransition (filled black circle) and that of the two resolved main transition components (blue circle and red triangle) are shown.
3.2. Relative enthalpy vs. composition plots
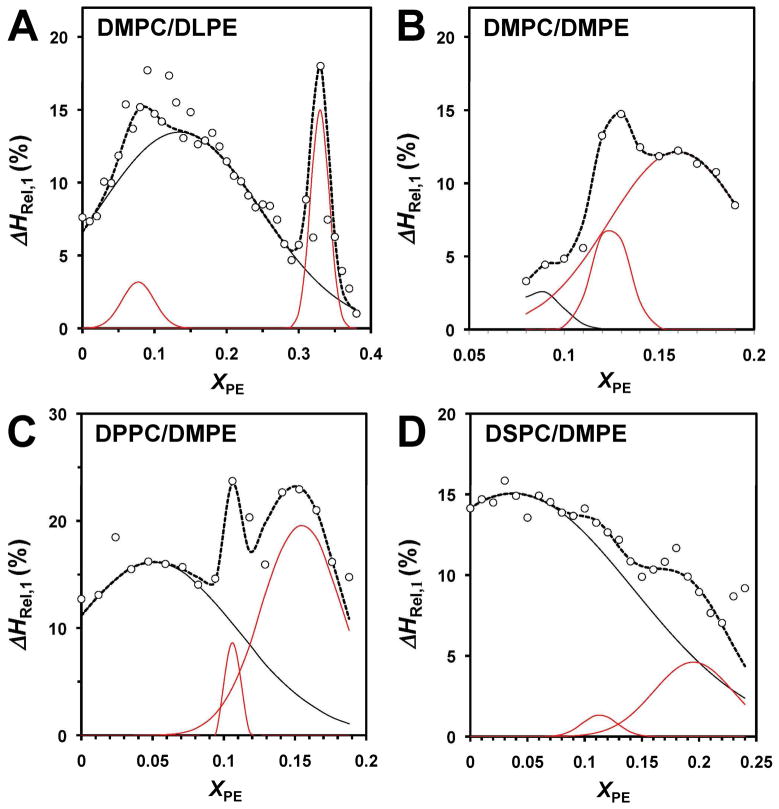
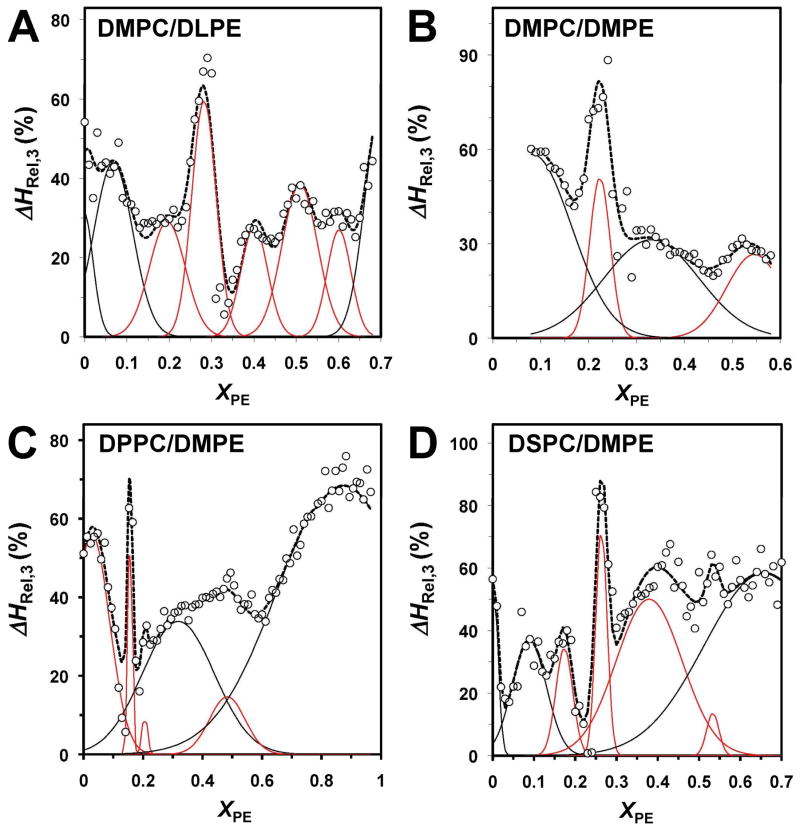
Figs. 5 and 6 show the relative enthalpies of the resolved pretransition (i =1) and the second main transition (i =3) components, i.e., ΔHRel,1 and ΔHRel,3, respectively, vs. XPE for the different mixtures of saturated PC and PE. As ΔHRel,2 = 100 − ΔHRel,1 − ΔHRel,3, plots of ΔHRel,2 are redundant and thus not shown. Since the resolved thermodynamics parameter vs. XPE plots indicated the presence of multiple overlapping peaks, Gaussian deconvolution was carried out. Among the resolved peaks only those (indicated by a red line) defined by more than two data points and of a reasonable width were considered relevant, while those (indicated by a black line) defined by one or two data points or unreasonably broad were rejected. The rejection of a broad deconvoluted peak was based on the criterion of FWHM of the resolved Gaussian Peak > X*PE (1−X*PE), where FWHM denotes the full width half maximum and X*PE the adjacent critical PE composition predicted by the SL-model.2 This “wide” peak rejection criterion, although somehow arbitrary, allowed us to take into account of the general trend of the greater separation between adjacent critical compositions with increasing PE.
Figure 5. Relative enthalpy of the pretransition (ΔHRel,1) vs. XPE for different PC/PE bilayers.
(A) DMPC/DLPE, (B) DMPC/DMPE, (C) DPPC/DMPE and (D) DSPC/DMPE. The data points indicated by open circles, the deconvoluted Gaussian peaks by a red line and their sum by a dotted line. The Gaussians drawn in black were rejected because they did not fit the criteria of acceptance (see text).
Figure 6. Relative enthalpy of the second resolved main transition component (ΔHRel,3) vs. XPE for different PC/PE bilayers.
(A) DMPC/DLPE, (B) DMPC/DMPE, (C) DPPC/DMPE and (D) DSPC/DMPE. See the legend of Fig. 5 for details.
In case of DMPC/DLPE bilayers, peaks at XPE of ~ 0.08 and 0.33 were observed in the ΔHRel,1 plot (Fig. 5A) and at ~ 0.2, 0.28, 0.4, 0.51 and 0.6 in the ΔHRel,3 plot (Fig. 6A). Several peaks in the ΔHRel,1 and ΔHRel,3 plots were also observed for the other PC/PE mixtures (Figs. 5 and 6) and are summarized in Table 2. Beside the saturated systems, we also studied the DSPC/DEPE bilayers in which the acyl chains of PE have trans-double bonds. No pretransition was observed for this system and thus only the main transition was analyzed. The plot of the relative enthalpy ΔHRel,3 showed peaks at XPE of ~0.21 and 0.25 (Fig. S5 in Supporting Information).
Table 2.
Critical XPE’s based on deviations (or merger) of the Tp and Tm plots and the peaks (± half width) of the 2-state transition enthalpy (ΔH2S,1(p), ΔH2S,2(m), ΔH2S,3(m)) and relative enthalpy (ΔHRel,1(p), ΔHRel,3(m)) plots associated with the pre- (p) and main transitions (m). Predicted critical XPE’s from the SL model of rectangular (R), center-rectangular (CR) and hexagonal (HX) symmetry are shown.
| XPER,CR,HX | Tp/Tm | ΔH2S,1(p) | ΔH2S,2(m) | ΔH2S,3(m) | ΔHRel,1(p) | ΔHRel,3 (m) |
|---|---|---|---|---|---|---|
| 0–0.111R,CR,HX | 0.084±0.0541 | 0.077±0.0651 | 0.077±0.0711 | |||
| 0.111CR,HX | 0.111±0.0992 | 0.108±0.0093 | ||||
| 0.113±0.0364 | ||||||
| 0.125CR,R | 0.154±0.0304 | 0.125±0.0222* | ||||
| 0.143CR,HX | ||||||
| 0.167R | 0.172±0.1111 | 0.158±0.0163 | 0.178±0.0412 | 0.160±0.0852 | 0.159±0.0183 | |
| 0.174±0.0503 | 0.155±0.0673 | 0.173±0.0484 | ||||
| 0.172±0.0865 | ||||||
| 0.200CR | 0.2002* | 0.215±0.0774 | 0.209±0.0432* | 0.210±0.0294* | 0.195±0.0824* | 0.196±0.1001* |
| 0.197±0.0824* | 0.224± 0.0512 | |||||
| 0.2003* | 0.208±0.0223* | |||||
| 0.214±0.0115 | ||||||
| 0.250R,CR,HX | 0.2504* | 0.254±0.0571* | 0.257±0.0591* | 0.264± 0.0324 | ||
| 0.265±0.0942 | 0.248± 0.0625* | |||||
| 0.257±0.1283* | ||||||
| 0.266±0.0835 | ||||||
| †0.286CR,HX | 0.288±0.0291* | 0.281±0.0531* | 0.282±0.0681* | |||
| 0.333CR, HX | 0.315±0.0081 | 0.312±0.1192 | 0.331±0.0531* | 0.329±0.0301* | ||
| 0.305±0.0504 | 0.326±0.1214* | |||||
| †0.400CR | 0.3801 | 0.370±0.0681 | 0.408±0.1061* | 0.401±0.0981* | ||
| 0.372±0.1522 | 0.379± 0.1884 | |||||
| 0.396±0.1183* | ||||||
| 0.427±0.1674 | ||||||
| 0.367±0.2295 | ||||||
| 0.500R | 0.467±0.0552 | 0.509±0.0991* | ||||
| 0.483±0.0414 | 0.489±0.1433* | |||||
| 0.534±0.0354 | ||||||
| 0.546±0.1372 | ||||||
| †0.600CR | 0.599±0.0443* | 0.601±0.0671* | ||||
| 0.667CR,HX | 0.689±0.1751 | |||||
| 0.677±0.0364 | ||||||
| 0.857CR,HX | 0.846±0.0643 | |||||
| 0.875CR,R |
Superscripts 1 to 5 correspond to DMPC/DLPE, DMPC/DMPE, DPPC/DMPE, DSPC/DMPE and DSPC/DEPE bilayer, respectively. The predicted critical XPE’s that are separated by less than 0.01 in the range of 0.2 ≤ XPE < 0.7 are indicated by superscript *. The observed critical XPE that does not match the predicted XPEs within ± 0.03 is in bold. The predicted critical XPE’s of 0.286, 0.400 and 0.600 indicated by superscript † correspond to superlattices of dimeric guests, i.e., 2 × 0.143, 2 × 0.200 and (1 – 2 × 0.200), respectively. See Materials and Methods for details.
3.3. Two-state transition enthalpy vs. composition plots
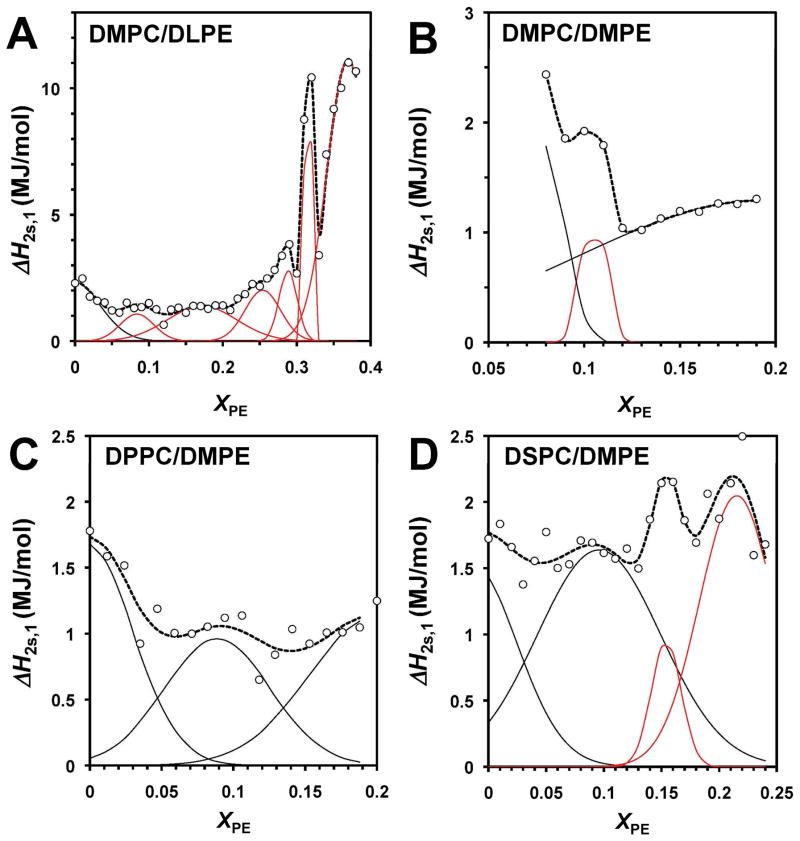
To obtain additional evidence on the presence of “critical” compositions we determined the two-state transition enthalpies (ΔH2S,i) of the resolved transitions and plotted them as a function of XPE. ΔH2S,i is a measure of the cooperativity of a phase transition (see Materials and Methods and S1 of Supporting Information). Fig. 7 shows the 2-state enthalpy (ΔH2S,1) of the pretransition vs. XPE for the different PC/PE mixtures. Clear peaks are observed in all mixtures except DPPC/DMPE. Some peaks are also observed in the ΔH2S,2 and ΔH2S,3 plots (Figs. 8 and 9). For the DSPC/DEPE system, peaks at ~ 0.17, 0.27 and (possibly) at 0.37 were observed in the ΔH2S,3 vs. XPE plot (Fig. S5 in Supporting Information). The XPE values of the indicated peaks are summarized in Table 2.
Figure 7. Two-state transition enthalpy of the pretransition (ΔH2S,1) vs. XPE for different PC/PE bilayers.
See the legend of Fig. 5 for details.
Figure 8. Two-state transition enthalpy for the first resolved component of the main transition (ΔH2S,2) vs. XPE for different PC/PE bilayers.
(A) DMPC/DLPE, (B) DMPC/DMPE, (C) DPPC/DMPE and (D) DSPC/DMPE. See the legend of Fig. 5 for details.
Figure 9. Two-state transition enthalpy for the second resolved component of the main transition (ΔH2S,3) vs. XPE for different PC/PE bilayers.
(A) DMPC/DLPE, (B) DMPC/DMPE, (C) DPPC/DMPE and (D) DSPC/DMPE. See the legend of Fig. 5 for details.
We also determined the size of the cooperative unit, i.e., NC,i = ΔH2S,i/ΔHi as a function of XPE for DMPC/DLPC bilayers. Sharp and prominent peaks were observed at ~ 0.33, 0.28 and 0.33 for the pretransition and the two main transition components, respectively (Fig. S6 in Supporting Information). Probable negative peaks were observed at ~ 0.2, 0.3, 0.4 and 0.5. See Discussion for the relevance of such negative peaks. All these compositions agree favorably with the critical compositions predicted by the SL-model.2
3.4. Correlation between the observed and the predicted “critical” compositions
In order to quantitatively evaluate the significance of Table 2, a rigorous statistical analysis using binomial distribution23 was performed (see Supporting Information). The analysis included eight predicted critical points within the range of XPE = 0.18 − 0.70, i.e. 0.200, 0.250, 0.286, 0.333, 0.400, 0.500, 0.600 and 0.667. This composition range includes 10% outside the smallest and largest critical values shown above. Values below XPE of 0.18 were excluded because the spacings between the predicted critical compositions are less that 2 mol% (twice the probable error of ~ 1 mole% in our sample composition) used in this study. The XPE values above 0.677 were excluded because of only a few samples were in that composition range.
As shown in Table 2, 47 critical compositions were observed experimentally at 0.18 < XPE < 0.7. Among these, 27 compositions deviated by less 1 mol% and 33 compositions by less than 2 mol% from the closest predicted critical composition. Statistical analysis based on the binomial distribution model23 (see Supporting Information) showed that the probability that 27 observed critical values would lie within ± 1 mol % of eight predicted ones just by random chance is one out of 1.3 × 104. In other words, it is highly unlikely that so many experimentally observed values would lie so close to the predicted ones simply by random chance. The concurrence of the experimental and predicted critical compositions is visualized in Fig. S7 of Supporting Information. Notably, this figure also shows that the observed critical compositions are more frequent and more closely spaced at low PE mole fractions, as predicted by the SL-model2,10,19. In conclusion, these statistical analyses strongly support the notion that multiple, SL-like arrangements of the component lipids are sequentially formed in PE/PC bilayers when the composition is varied.
4. DISCUSSION
The present study shows that the calorimetric behavior of binary PC/PE bilayers is complex, i.e. multiple transition components are detected. Beside the pre-transition, the main transition typically consists of two components (Figs. 1 and 2). To account for this complex behavior, we employed a multiple 2-state transition model assuming the presence of independent transition components defined by unique set of thermodynamics parameters. Each ith component is characterized by the resolved transition temperature (Ti), relative enthalpy (ΔHRel,i) and 2-state transition enthalpy (ΔH2s,i). The multiple 2-state transition model represents a convenient way to analyze complex systems like PE/PC bilayers. While this model probably oversimplifies the situation by assuming that coexisting domains undergo the transition independently, models accounting for putative interdomain interactions are too complex to co be implemented here.
Our results indicate that some or all of the resolved thermodynamics parameters change abruptly at particular compositions. Intriguingly, these compositions typically agree closely to “critical” compositions predicted by the SL-model proposing that head group of the guest lipid tend to adopt regular lateral distribution with a rectangular, centered-rectangular and hexagonal symmetry in the host lipid lattice2,10,19. The SL-model states that when the mole fraction of the guest increases, (i) multiple superlattices with increasing guest density are formed sequentially and (ii) the bilayer area covered by a particular superlattice peaks at each critical composition.2 Because lipid packing in superlattice domains is predicted and also indicated by MD simulations24 to be tighter than in domains of random organization, the transition enthalpy and cooperativity in the former are expected be higher than in the latter. As shown in Table 2, the observed maxima of ΔH2s,i and/or ΔHRel,i nearly always coincide within 1–2 mol% with predicted critical compositions. Statistical analysis (see Supplementing Information) indicated that the probability for fortuitous agreement between so many observed and predicted critical compositions is very low. Accordingly, the present calorimetric data provides strong support for that multiple different superlattices can be present in gel-state PC/PE bilayers.
Unlike the transition enthalpy, the size of the co-operative unit does not need to correlate with the mode of lateral organization (superlattice versus random). Tighter packing of the lipids in superlattice domains may reduce the bilayer curvature as compared to random domains of similar composition thus increasing the size of the cooperative unit. Alternatively, tighter packing in superlattice domains could also result in increased curvature, thus reducing the size of the cooperative unit as compared to random domains. Accordingly, depending on the ratio of the components in a particular superlattice and detail on the constituent molecules, the size of the cooperative unit could be higher, equal or smaller than that of random domains of similar composition. Thus the finding that the cooperativity vs. compositions plots (Fig. S6 in Supporting Information) appears to display both maxima and minima coinciding with predicted critical compositions is not unexpected.
Not all predicted critical compositions were observed in each PC/PE mixture. For instance, the critical XPE of 0.286 was observed only for the DMPC/DLPC mixture, while those at 0.25 and 0.33 were observed for several PC/PE mixtures (Table 2). There could be several reasons for this. First, the stability of the predicted superlattices may vary significantly and thus those of lesser stability could pass unnoticed. The stability of a particular superlattice depends on the interactions between the component lipids, which could be highly sensitive to minor variations in structure. Notably, our previous analysis of calorimetric data published by others 25,26 showed that the effect of cholesterol on the transition enthalpy of phospholipids is highly sensitive to the acyl chain length, as shown by the fact that the number of perturbed acyl chains in the second layer proximal to cholesterol increases from 1 to 9 when the acyl chain length decreases from 20 to 14 carbons.27 Also the previous calorimetric studies on PC/PE bilayers have shown that the phase behavior is highly sensitive to the acyl chain length of the components.11,28
The critical XPE’s at 0.286, 0.40 and 0.600 are not predicted by the original SL-model assuming the presence of monomeric quest only.2,3,19 However, they can be explained by assuming the formation of dimeric guest as shown in Fig. S7C in Supporting Information. The dimeric guest would be PE at XPE’s of 0.286 and 0.40 and PC at XPE of 0.60. The reasons for the presence of putative guest dimers are not obvious, but could relate to the intermolecular hydrogen bonding between PE molecules.29 Such hydrogen bonding could stabilize dimeric PE guest directly and dimeric PC guest indirectly, i.e. formation of a hydrogen bonded network among PE hosts could promote dimerization of PC molecules. The indicated critical composition at XPE ~ 0.546 is neither predicted by the monomeric quest model nor attributable to the formation of guest dimers. We speculate that it might be due to the fusion of the enthalpic peaks deriving from superlattices with XPE of 0.50 and 0.60.
In this study the total amount of phospholipid per sample was fixed at 1 μmol. We realize that this may results in missing some minor and/or very broad transitions. However, since we were primarily interested in identifying abrupt changes in the thermodynamics parameters over a narrow composition range, we opted to maintain a constant total lipid concentration. A more relevant concern is the composition of multilamellar liposomes, i.e. their true composition could deviate from the nominal one due to errors in pipetting the lipid mixtures. However, we have no reason to doubt that the actual compositions would differ more that ± 1 mol % from the nominal ones because (1) extra care was taken when pipetting the samples using precision syringes (see Materials and Methods), (2) our previous tests16 have shown that this accuracy can be achieved routinely and (3) Tm vs. composition plots do not show obvious signs of significant errors in composition (scatter). Another concern is that some liposome preparations could be inhomogeneous, i.e. consisting of population of different compositions. Since we did not detect any significant or measurable transition due to pure PE or PC in the mixed samples, complete or extensive segregation of the component lipids to different liposome populations is unlikely. However, it is not possible to fully exclude partial segregation. This issue might be resolved by carrying out extensive imaging and spectroscopic studies, but these are beyond the scope of this study. Nevertheless, we think that heterogeneity of the liposomes cannot explain our results, since extra care was taken to prevent component segregation when preparing the liposomes (see Materials and Methods). Most importantly, it is difficult to see how liposomal heterogeneity would results in multiple, abrupt changes in thermodynamic parameters and why these would occur at or close to the critical composition predicted by the SL-model.
The formation of superlattices in PC/PE bilayers probably relates to the complementary shapes of PC and PE. The cross-sectional area of the highly hydrated head group of PC is larger than the cross-sectional area of its acyl chains,30,31 which results in crowding at the head group level. Inclusion of PE, which has a much smaller head group, relieves such crowding when mixed with PC. This “spacer effect” is obviously maximal when PE is evenly distributed in the PC matrix and thus drives the formation of SL-like arrangements.2
The lack of significant hysteresis indicated by the similarity of the heating and cooling scans implies that the tendency to adopt SL-like lateral arrangements is maintained in the liquid-crystalline state as well. However, due to the diminished lateral packing in this state, the size and stability of superlattice domains are likely to significantly diminish. Existence of SL-like domains in liquid crystalline PC/PE bilayers is supported by previous fluorescence and FTIR data.7–9,32 Notably, while long-range order is unlikely to exist in the liquid-crystalline state, a high degree of local lateral order can exist as found previously for the smectic A′ phase.5
We have previously proposed that superlattice formation could play a key role in the regulation of the composition of biological membranes2,3. While most of the data supporting this idea comes from studies on model membranes, it is intriguing that the phospholipid compositions of the erythrocyte and platelet membranes from several mammalian species coincide remarkably well with the critical compositions predicted by the superlattice model19. Since calorimetry mainly provides information on the gel-state, it is not straightforward to project the present results on natural membranes, which are typically in the liquid-crystalline or liquid-order state. However, it seems feasible that the superlattice organization in the gel state is maintained above the phase transitions, i.e. in the liquid crystalline state, albeit over a shorter length scale. Supporting this, spectroscopic studies on liquid-crystalline PE/PC bilayers have revealed similar critical compositions as in the present study.7–10 It is also notable that some biological membranes or similar lipid assemblies could be in the gel (or similar) state at the physiological temperature. The best example is stratum corneum, the outermost layer of mammalian skin, in which the lipids are laterally highly ordered as shown by diffraction studies33. It seems likely that the lipids in stratum corneum adopt a SL-like lateral organization. It is also probable that rafts or similar liquid-order domains have a superlattice-like lateral organization of a short range.
5. Conclusions
In conclusion, this noninvasive DSC study provides evidence that the molecules in binary PC/PE bilayers tend to adopt regular, SL-like lateral arrangements, which could be involved in the regulation of the lipid compositions of biological membranes.
Supplementary Material
Acknowledgments
This work was supported by the Robert A. Welch Research Foundation grant (D-1158), Williams endowment fund of Trinity University and the NIH grant (RC1-GM090897-02) given to K.H.C and grants from the Finnish Academy and Sigrid Juselius Foundation to P.S.
Footnotes
SUPPORING INFORMATION AVAILABLE
Additional information on the simulations of multiple 2-state transition parameters and cooperativity (S1), the DSC scans of DMPC/DMPE (S2), the comparisons of heating, cooling and slow scans of DMPC/DLPE bilayers (S3), the chi-square values vs. composition plots (S4), the resolved thermodynamics parameters on DSPC/DEPE bilayers (S5), the estimation of the number of a cooperative unit in DMPC/DLPE bilayers (S6), the graphical comparison of predicted and observed critical compositions (S7), and the statistical analysis of the distribution of experimental versus predicted critical compositions using the binomial distribution model (S8) are given in Supporting Information. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Simons K, Gerl MJ. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 2.Somerharju P, Virtanen JA, Cheng KH, Hermansson M. Biochim Biophys Acta. 2009;1788:12–23. doi: 10.1016/j.bbamem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Somerharju P, Virtanen JA, Cheng KH. Biochim Biophys Acta. 1999;1440:32–48. doi: 10.1016/s1388-1981(99)00106-7. [DOI] [PubMed] [Google Scholar]
- 4.Chong PL, Zhu W, Venegas B. Biochim Biophys Acta. 2009;1788:2–11. doi: 10.1016/j.bbamem.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Chou CF, Jin AJ, Hui SW, Huang CC, Ho JT. Science. 1998;280:1424–1426. doi: 10.1126/science.280.5368.1424. [DOI] [PubMed] [Google Scholar]
- 6.Hermansson M, Hokynar K, Somerharju P. Prog Lipid Res. 2011;50:240–257. doi: 10.1016/j.plipres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Cheng KH, Virtanen J, Somerharju P. Biophys J. 1999;77:3108–3119. doi: 10.1016/S0006-3495(99)77141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng KH, Ruonala M, Virtanen J, Somerharju P. Biophys J. 1997;73:1967–1976. doi: 10.1016/S0006-3495(97)78227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng KH, Cannon B, Metze J, Lewis A, Huang J, Vaughn MW, Zhu Q, Somerharju P, Virtanen J. Biochemistry. 2006;45:10855–10864. doi: 10.1021/bi060937y. [DOI] [PubMed] [Google Scholar]
- 10.Cannon B, Lewis A, Metze J, Thiagarajan V, Vaughn MW, Somerharju P, Virtanen J, Huang J, Cheng KH. J Phys Chem B. 2006;110:6339–6350. doi: 10.1021/jp0558371. [DOI] [PubMed] [Google Scholar]
- 11.Sugar I, Monticelli G. Biophys Chem. 1983;18:281–289. doi: 10.1016/0301-4622(83)80041-6. [DOI] [PubMed] [Google Scholar]
- 12.Silvius JR, Brown PM, O’Leary TJ. Biochemistry. 1986;25:4249–4258. doi: 10.1021/bi00363a012. [DOI] [PubMed] [Google Scholar]
- 13.Mabrey S, Sturtevant JM. Proc Natl Acad Sci USA. 1976;73:3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blume A, Wittebort RJ, Das Gupta SK, Griffin RG. Biochemistry. 1982;21:6243–6253. doi: 10.1021/bi00267a032. [DOI] [PubMed] [Google Scholar]
- 15.Blume A, Ackermann T. FEBS letters. 1974;43:71–74. doi: 10.1016/0014-5793(74)81108-7. [DOI] [PubMed] [Google Scholar]
- 16.Haimi P, Hermansson M, Batchu KC, Virtanen JA, Somerharju P. J Biol Chem. 2010;285:751–760. doi: 10.1074/jbc.M109.061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubenstein JLR, Owicki JC, McConnell HM. Biochemistry. 1980;19:569–573. doi: 10.1021/bi00544a027. [DOI] [PubMed] [Google Scholar]
- 18.Cooper A, Nutley MA, Wadood A. In: Protein-ligand interactions: hydrodynamics and calorimtery. Harding SE, Chowdhry BZ, editors. Oxford University Press; New York: 2001. pp. 289–318. [Google Scholar]
- 19.Virtanen JA, Cheng KH, Somerharju P. Proc Natl Acad Sci U S A. 1998;95:4964–4969. doi: 10.1073/pnas.95.9.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small DM. In: Handbook of Lipid Research. Hanaham DK, editor. Plenum Press; New York: 1986. pp. 97–143. [Google Scholar]
- 21.Lentz BR, Freire E, Biltonen RL. Biochemistry. 1978;17:4475–4480. doi: 10.1021/bi00614a018. [DOI] [PubMed] [Google Scholar]
- 22.Black SG, Dixon GS. Biochemistry. 1981;20:6740–6744. doi: 10.1021/bi00526a033. [DOI] [PubMed] [Google Scholar]
- 23.Mendenhall W, Sincich T. Statistics for Engineering and the Sciences. Dellen Publishing Co; San Francisco: 1992. [Google Scholar]
- 24.Zhu Q, Cheng KH, Vaughn MW. J Phys Chem B. 2007;111:11021–11031. doi: 10.1021/jp070487z. [DOI] [PubMed] [Google Scholar]
- 25.McMullen TPW, McElhaney RN. Biochemistry. 1997;36:4979–4986. doi: 10.1021/bi962815j. [DOI] [PubMed] [Google Scholar]
- 26.McMullen TPW, Lewis RNAH, McElhaney RN. Biochemistry. 1993;32:516–522. doi: 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- 27.Virtanen JA, Somerharju P. J Phys Chem. 1999;103:10289–10293. [Google Scholar]
- 28.Sugar IP, Monticelli G. Biophys J. 1985;48:283–288. doi: 10.1016/S0006-3495(85)83781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh R. Biochemistry. 1988;27:7750–7758. doi: 10.1021/bi00420a025. [DOI] [PubMed] [Google Scholar]
- 30.McIntosh TJ. Biophys J. 1980;29:237–245. doi: 10.1016/S0006-3495(80)85128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Israelachvili JN, Mitchell DJ. Biophys Biochim Acta. 1975;389:13–19. doi: 10.1016/0005-2736(75)90381-8. [DOI] [PubMed] [Google Scholar]
- 32.Cannon B, Lewis A, Metze J, Thiagarajan V, Vaughn MW, Somerharju P, Virtanen J, Huang J, Cheng KH. J Phys Chem B. 2006;110:6339–6350. doi: 10.1021/jp0558371. [DOI] [PubMed] [Google Scholar]
- 33.Bouwstra JA, Ponec M. Biochim Biophys Acta. 2006;1758:2080–2095. doi: 10.1016/j.bbamem.2006.06.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.