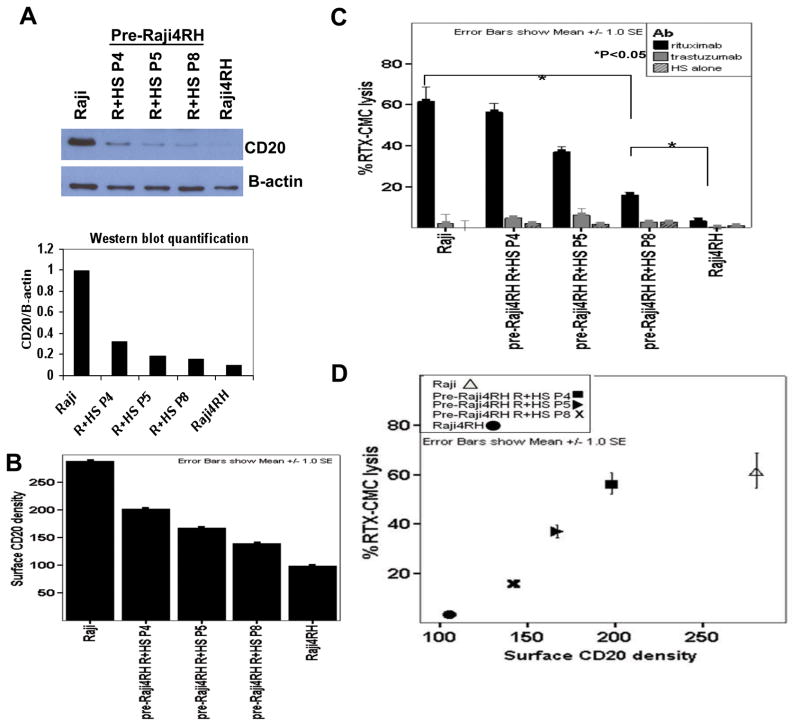
Figure 1. Gradual reduction of CD20 expression was observed during the development of rituximab-resistant cell lines (RRCLs).
Raji, rituximab-sensitive cell line, was exposed to repeated, escalating doses of rituximab plus human serum (R+HS) before the rituximab-resistant cell line (RRCL), Raji4RH, was generated at the final 10th passage. Various passages (abbreviated as “P”) were collected during the process of RRCL development and were analyzed. (A) Total CD20 protein expression was analyzed for passages 4, 5, 8 (P4, P5, P8), the final passage (Raji4RH)and the parentalcell line, Raji, by Western blot. (B) Surface CD20 density at different passages were analyzed by Amnis ImageStream technology and calculated according to the following formula: Surface CD20 density=mean surface CD20-FITC intensity/mean surface area (CD20-FITC/μm2). (C) The percentage of rituximab-associated CMC in each passage was determined by 51Cr release assay. (D) Decreasing surface CD20 density was directly correlated with loss of rituximab-associated complement-mediated cytotoxicity (rituximab-CMC).