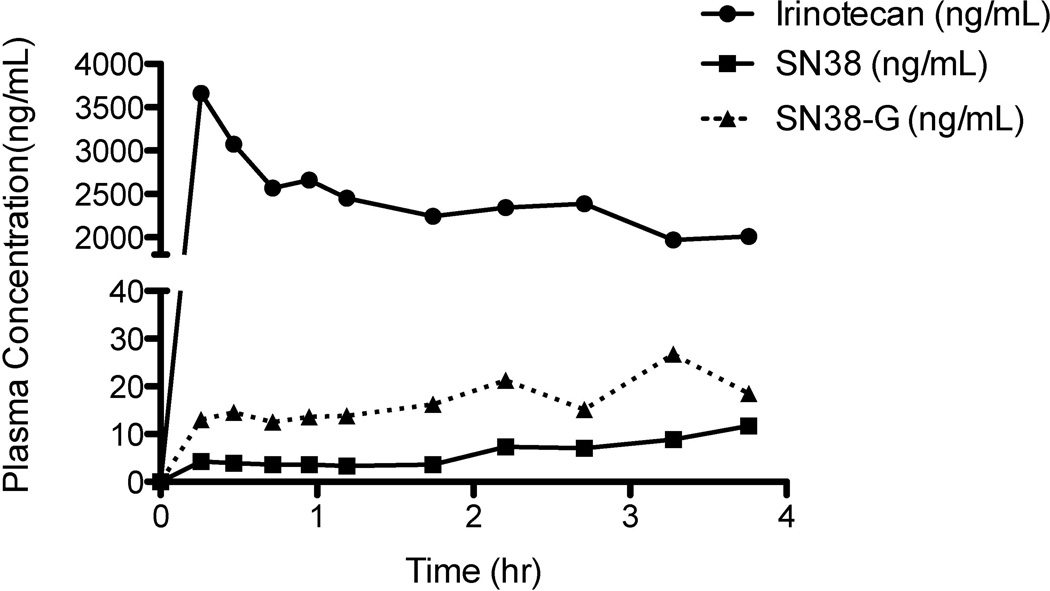
Figure 3.
Preclinical plasma concentration versus time curves for irinotecan, SN38, and SN38-G from preclinical TACE experiments performed on a pig. The porcine model demonstrates a very high irinotecan maximum plasma concentration (Cmax), much higher than the upper limit of quantification for irinotecan (1000 ng/mL). This validates the need for the dilution QC at 5000 ng/mL for irinotecan only.