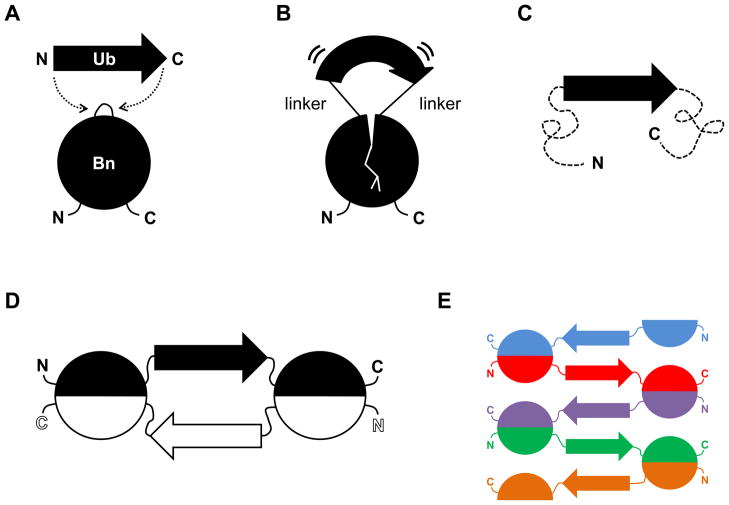
Figure 1.
Schematic of mutually exclusive folding-induced domain swapping. (A) Ub (38 Å N-to-C distance) is inserted into a surface loop of Bn (10 Å distance between the termini of the loop at position 66). (B) If the linkers used to join Ub and Bn are sufficiently short, the Ub domain stretches the Bn domain and the Bn domain compresses the Ub domain. (C) The more stable Ub domain stretches the Bn domain to the point where it unfolds (dashed lines). This state is expected to be stable only at low protein concentrations. (D) The Bn domain refolds by domain swapping with an identical monomer to generate a closed dimer. This species is anticipated to be populated at intermediate protein concentrations. (E) Runaway swapping is predicted to occur at high protein concentrations, producing a long, linear polymer.