Figure 4.
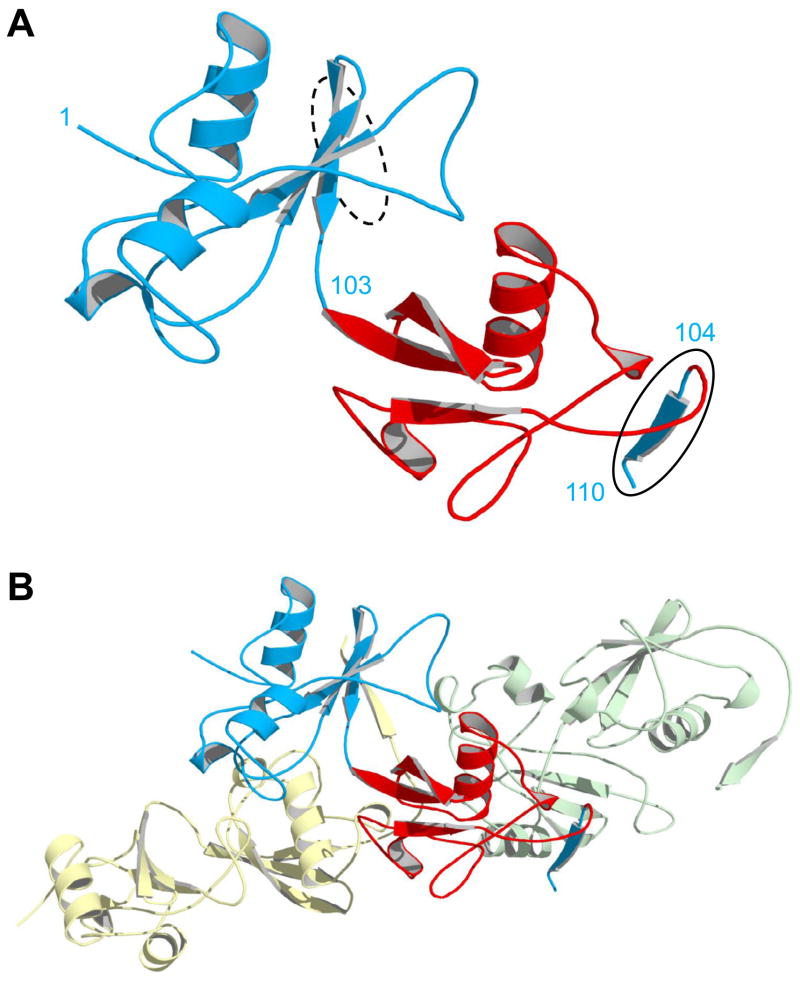
X-ray structure of domain-swapped BU103. (A) The asymmetric unit consists of a single monomer in which the Ub domain (red) has split apart the Bn domain (blue). Residues are numbered according to the Bn sequence. Bn residues 1–103 and 104–110 (solid oval) extend from the N- and C-termini of Ub at left and right, respectively. The dashed oval indicates where residues 104–110 would normally be located in WT Bn, as the fifth strand of the central β-sheet. (B) The same monomer in panel A is now shown with the molecules in the adjacent asymmetric units. Bn residues 104–110 of the central monomer (blue) insert into the βsheet of the next protomer (green), and the residues 104–110 from the preceding molecule (yellow) complex with residues 1–103 of the central monomer. In this arrangement, all native Bn interactions are restored and conformational strain is relieved.
BU103 was crystallized at 20 °C using the hanging-drop vapor-diffusion method with the mother liquor consisting of 10 mM Tris (pH 8.0), 1 M (NH4)2SO4, 1.5 % isopropanol (v/v). X-ray diffraction data were collected at station A1 at the Macromolecular Diffraction Facility at the Cornell High Energy Synchrotron Source (MacCHESS), reduced using HKL-2000 (25), phased by molecular replacement (1UBQ and residues 3–103 of chain A of 1A2P), and refined using Phenix (26). X-ray statistics are listed in Supplementary Table S1.