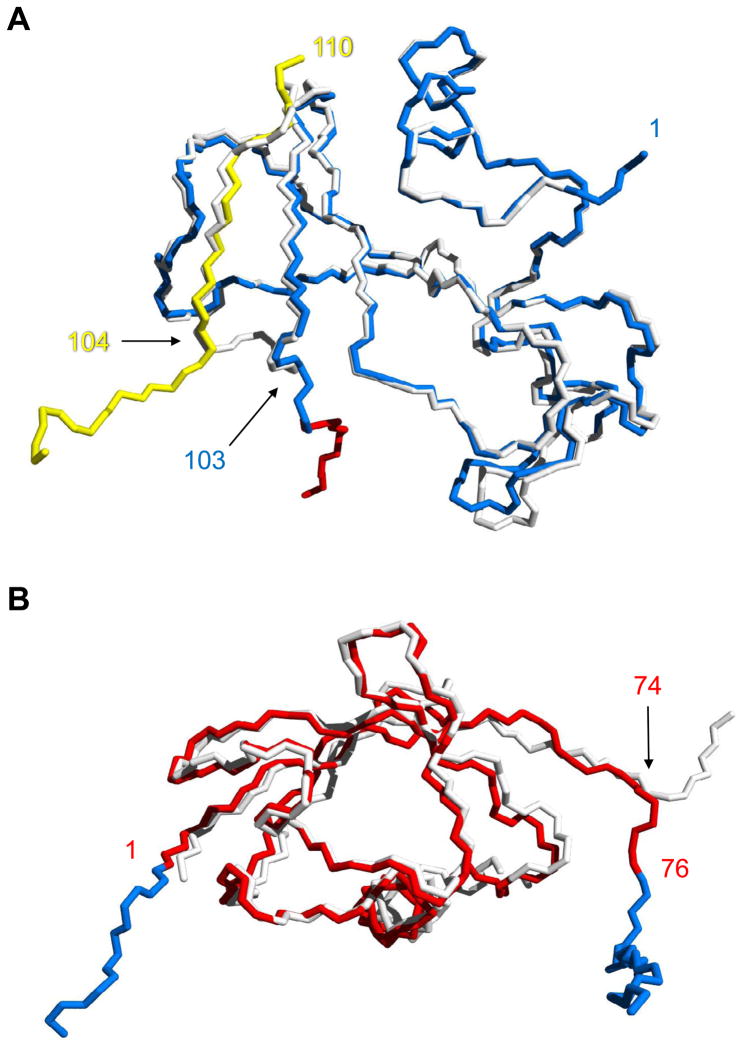
Figure 5.
Alignment of the Bn and Ub domains of BU103 with their respective WT proteins. (A) Comparison of inter- and intramolecularly folded Bn. Backbone atoms of the Bn domain of BU103 (blue and yellow) are superimposed on those of WT Bn (white). Residues are numbered according to the Bn sequence. The point of strand exchange is marked by residue 103 from the blue chain and residue 104 from the protomer in the preceding asymmetric unit (yellow chain). The red residues signify the beginning of the Ub domain. (B) Alignment of the Ub domain of BU103 (red) with WT Ub (white). Residues are numbered according to the Ub sequence. Bn fragments 1–103 and 104–110 (blue) extend from the N-terminus (residue 1) and C-terminus (residue 76) of the Ub domain, respectively.