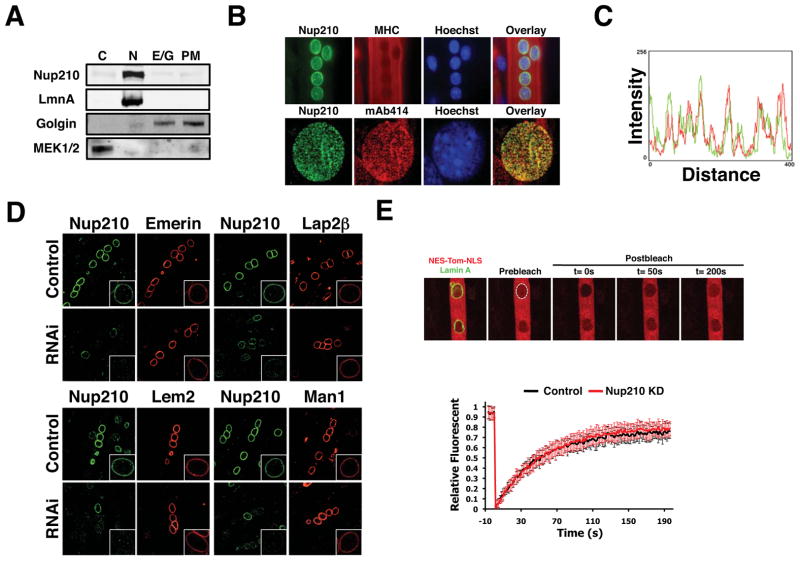
Figure 3. NPC-associated Nup210 does not affect INMP targeting or global nucleo-cytoplasmic transport.
(A) Post-mitotic myotubes were fractionated by sequential centrifugation and Nup210 protein leveles were analyzed in the different subcellular fractions by western blot (C, Cytoplasm; N, nucleus; E/G, Endoplasmic Reticulum/Golgi; PM, Plasma Membrane). (B) Nup210 localization in post-mitotic myotubes was analyzed by immunofluorescence. MHC was used as a marker of differentiation and mAb414 as a marker for NPCs. (C) Co-localization of Nup210 and mAb414 signals at the nuclear envelope was determined using Image J. (D) C2C12 myoblasts were transfected with Emerin-V5, V5-Lap2B, Lem2-V5 and V5-Man1 and induced to differentiate. Cells were infected with lentivirus carrying control or Nup210 shRNAs at 36 hours after differentiation and stained for Nup210 and V5 at 96h post-infection. Inlets show higher magnification of myotube nuclei. (E) C2C12 myoblasts were transfected with NES-Tomato-NLS and Lamin A-EGFP and induced to differentiate. Cells were infected with lentivirus carrying control or Nup210 shRNAs at 36 hours after differentiation and nuclear transport was analyzed by FRAP of NES-Tomato-NLS 96h post-infection. Values represent the average of 3 different independent experiments ± SD. See also Figures S3 and S4.