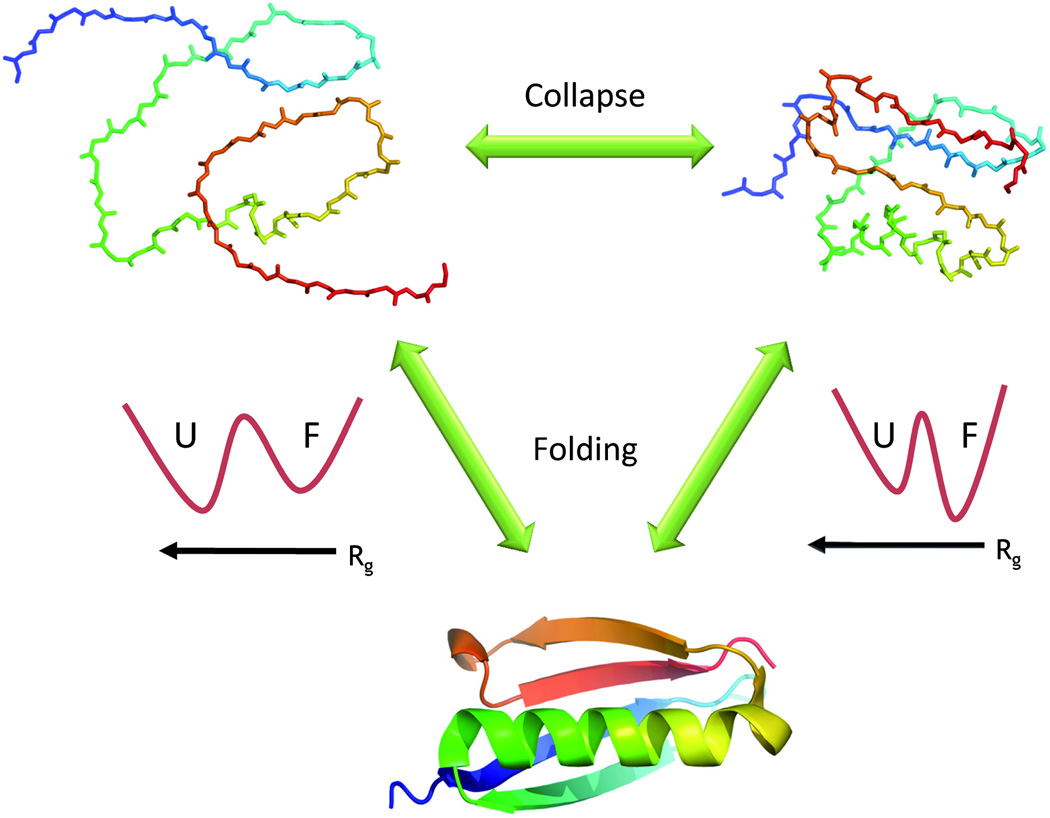
Figure 1. The collapse transition and protein folding.
Unfolded proteins may change their configuration depending on the quality of the solution. In a good solvent they are expanded (top left) while in a bad solvent they are collapsed (top right). The collapse transition has been observed in both equilibrium and time-resolved experiments. The configuration of the chain may have an effect on folding thermodynamics. Thus, an expanded chain (whose radius of gyration, Rg, is much larger than that of the folded protein) is stabilized with respect to the folded state due to its higher configurational entropy. This is depicted by the schematic free energy surface on the left (U- unfolded state, F- folded state), plotted as a function of Rg. When the protein collapses, it loses much of its configurational entropy, and it is therefore less stable than the folded state- see the free energy surface on the right. The entropic loss accompanying collapse may thus facilitate folding.