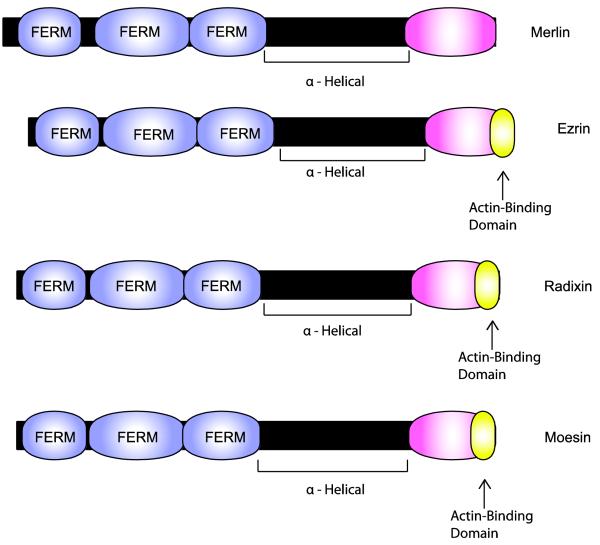
Fig. 8.
Schematic illustrating the functional domains present in merlin and comparing the structure of merlin to that of its closest relatives (ezrin, radixin and moesin). The lengths of each protein (indicated by the black bars) and domains within each of these proteins (indicated by the colored expansions) are scaled to the actual number of amino acids in each. Note that all four of these proteins contain a highly conserved amino terminal FERM domain composed of three related subdomains. In each of these proteins, the FERM domain is linked to a COOH domain by an α-helical region that allows these proteins to flex and facilitates intramolecular association between the FERM and COOH domains. As indicated in the text, this intramolecular association regulates the activity of these proteins. Curiously, however, merlin is apparently active in the “closed” configuration while this configuration is inactive for the other three proteins. A key signal regulating the configuration of these proteins is the phosphorylation of Ser518 in merlin and specific threonine residues in the COOH domain of ezrin, radixin and moesin. Ezrin, radixin and moesin all also contain a domain located at their extreme carboxy terminus that is capable of binding to filamentous (F) actin. This domain is not conserved in merlin.