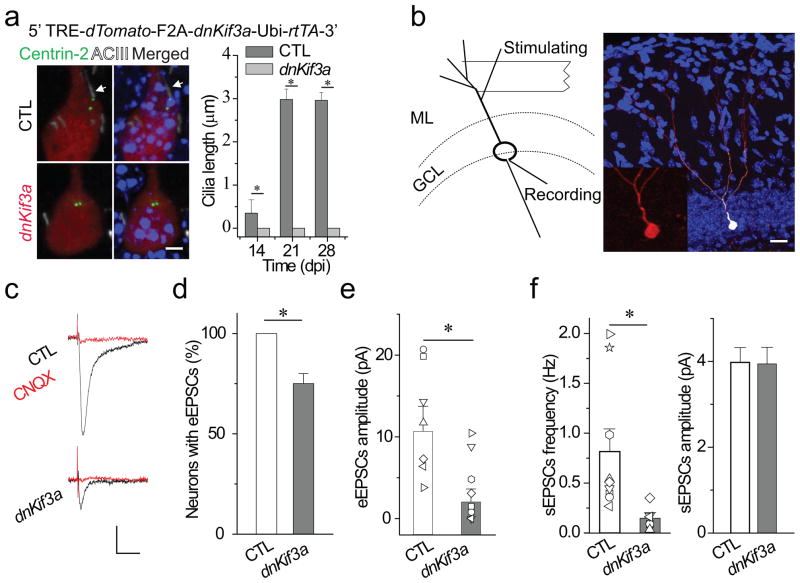
Figure 3. Primary cilia deletion caused by expressing dnKif3a severely disrupts functional glutamatergic synapse formation in adult-born neurons.
(a) Primary cilia deletion in adult-born DGCs by dnKif3a ectopic expression. At top, a diagram of the inducible retroviral vector (dnKif3a), below, typical adult-born DGCs labeled with markers of cilia (White, ACIII staining), centrioles (Green, Centrin-2-EGFP) and adult-born DGCs (Red, dTomato). Arrows point to centrosome. Scale bar: 5 μm.At right, a comparison of cilia length in control and dnKif3a+ adult-born DGCs. (b) A schematic diagram of the electrophysiological testing and an image of a recorded newborn DGC filled with biocytin through the recording pipette. The stimulating electrode was placed in the outer molecular layer to excite principally the entorhinal cortical projections. The inset is an enlarged image of the recorded adult-born DGC with dTomato signal. GCL, granule cell layer. ML, molecular layer. Scale bar: 25 μm. (c–f) Glutamatergic synaptic transmission recorded from control and dnKif3a+ adult-born DGCs at 21 dpi. c) Sample traces of glutamatergic synaptic transmission in the presence of 5 μ bicuculline. Cells were held at Vm=-65 mV. Scale bars: 10 pA and 15 ms. d) The percent of recorded newborn DGCs with detectable synaptic transmission. e) eEPSCs amplitude. Shown are individual and averaged eEPSCs amplitudes from control and dnKif3a+ adult-born DGCs. f) Spontaneous glutamateric synaptic transmission, frequency and amplitude. In a, d–f, all values represent mean±SEM (n=7–12 neurons; *: p<0.01, ANOVA).