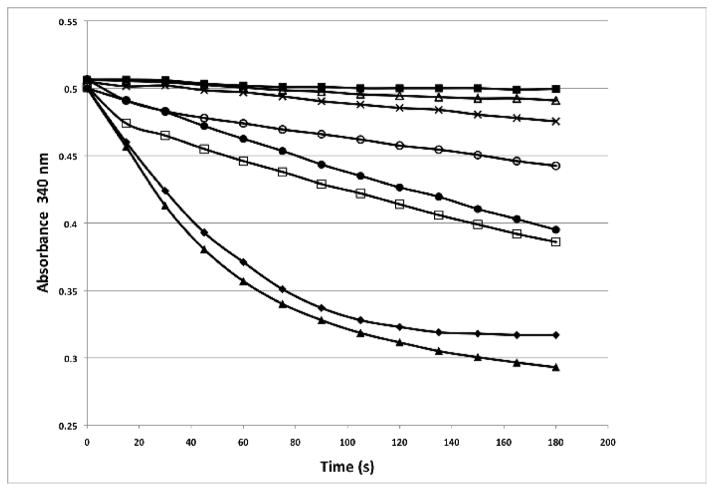
Fig. 2.
Comparison of MeCPA activity in partially purified enzyme preparations from different strains of E. coli. All samples were prepared in an identical manner, as detailed in the Materials and methods. Briefly, cultures expressing the MBP-proMeCPA-His6PR fusion protein, and in some cases also expressing wild-type or mutant DsbC or a DsbC/DsbA fusion protein, were induced with IPTG and grown overnight at 18 °C. The cells were pelleted by centrifugation, resuspended in buffer A, lysed, and subjected to amylose affinity chromatography. Equal amounts of the partially pure fusion proteins were treated with thermolysin and then assayed for carboxypeptidase activity by monitoring the decrease in absorbance at 330 nm that accompanies the hydrolysis of FAPP. (■), thermolysin only (no protein); (△), BL21(DE3) cells; (×), Origami B(DE3) cells; (○), T7 SHuffle Express cells; (●), T7 SHuffle Express cells + pBA2219; (▲), BL21(DE3) cells + pBA2219; (□), BL21(DE3) cells + pBA2219; (◆), BL21(DE3) cells + pBA2285.