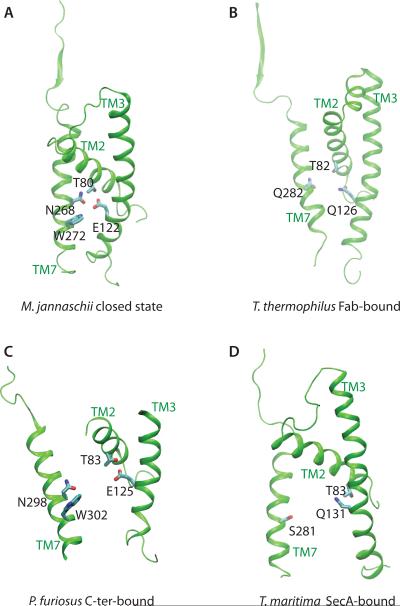
Figure 6.
H bonding in the central TM2-TM3-TM7 cluster of SecY is coupled to SecY's conformation. The TM2, TM3, and TM7 helices with selected amino acid residues are depicted for the M. jannaschii SecY translocon in the closed state [9] (A), and for the structures thought to represent SecY open to various extents: the Fab-bound T. thermophilus [50] (B), the archaeal P. furiousus bound to the C-terminal fragment of another SecY copy [51] (C), and the SecA-bound T. maritima translocon [49] (D). Note that in the various open structures TM2 and TM3 remain relatively close to each other, whereas TM7 moves away from TM2/TM3. M. jannaschii E122 is conserved as Glu in the archaeal P. furiosus SecY (panel C), but replaced by a Gln in the bacterial T. thermophilus and T. maritima translocons (panels B&D). S281 of T. maritima SecY is part of an array of Ser/Thr groups along TM7 [11]. In the closed state of the T. maritima SecY, the short sidechain of S281 and the presence of a Gln instead of a Glu at position 131 could make H bonding to T83/Q131 weaker than in the corresponding M. jannaschii cluster. That is, the extent to which the translocon opens and the kinetics of translocon opening may be different in archaeal vs. bacterial translocons.