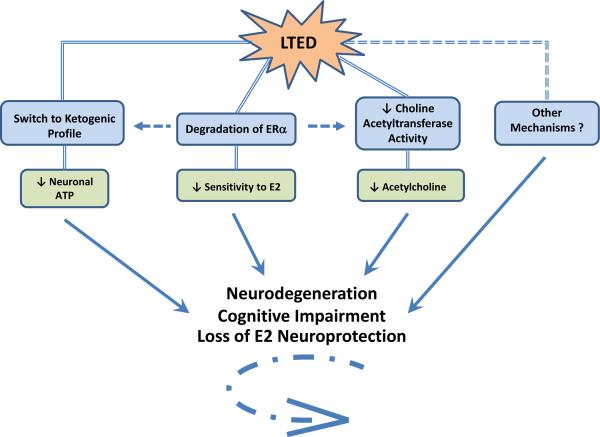
Figure 6. Neurological Consequences of Long-Term Estradiol Deprivation.
This figure summarizes the mechanisms currently thought to underlie the three major neurological consequences of long-term E2 deprivation (LTED): neurodegeneration, cognitive impairment, and loss of E2 neuroprotection. Brinton and others have shown that E2 maintains favorable mitochondrial bioenergetics in neurons, facilitating their use of glucose as the primary source of energy through oxidative phosphorylation [195]. However, during LTED, E2 is not able to serve its purpose, and neurons begin to switch their preferred energy source from glucose to ketones [195]. Ketones are a much less efficient source of energy for neurons, and their usage leads to an overall decreased amount of available ATP. Secondly, our lab recently demonstrated, for the first time, that the unbound portion of E2's cognate receptor, ERα, is degraded in the hippocampus during LTED [202]. This leads to hippocampal-specific insensitivity to E2 and loss of E2 neuroprotection against cerebral ischemia. Finally, E2's ability to enhance attentional processes, long-term potentiation, dendritic spine density, and the activity of choline acetyltransferase, the rate-limiting enzyme for synthesis of the neurotransmitter acetylcholine (ACh), is lost during LTED [12]. A lack of ACh hinders learning and memory and is closely linked with cognitive impairment and Alzheimer's disease. Additionally, there may be some cross-talk between these major events that occur during LTED, as evidenced by connecting arrows. See text for more details. E2, 17β-estradiol; ERα, Estrogen Receptor Alpha; ATP, Adenosine Triphosphate.